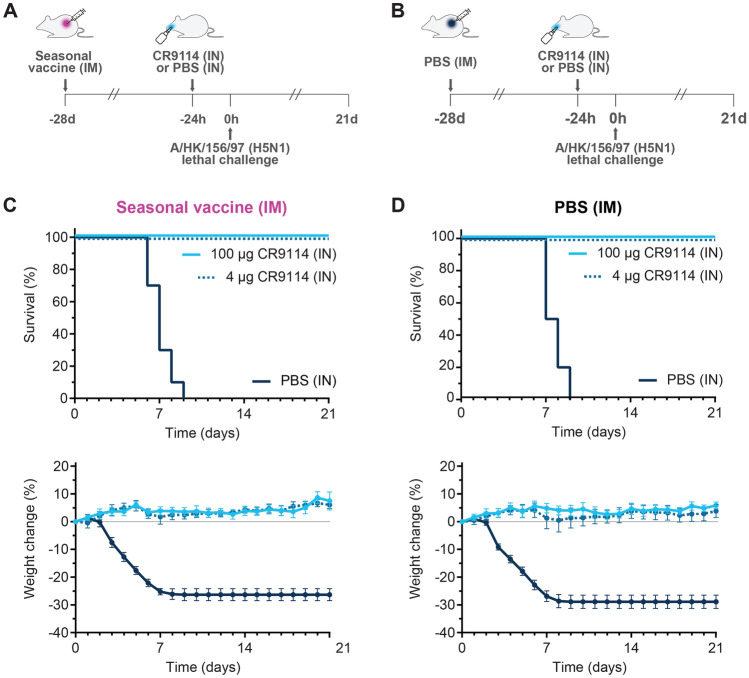
Figure 2.
Prophylactic intranasal administration of CR9114 protects mice against A(H5N1), both in the absence and in the presence of pre-existing immunity elicited by the seasonal influenza vaccine. Schematic depiction of the study design testing the prophylactic efficacy of intranasally administered CR9114 versus PBS (-24 h) in mice immunized intramuscularly with Influvac® quadrivalent vaccine (A), or PBS at 28 days before challenge (B). Percentages of survival (top panels) and weight change (bottom panels) of mice challenged with A/HK/156/97 (H5N1) 24 h after intranasal administration with 4 µg CR9114 (0.2 mg/kg assuming each mouse weights 20 g), 100 µg CR9114 (5 mg/kg assuming each mouse weights 20 g) or PBS in the (C) presence of pre-existing immunity from intramuscular Influvac® quadrivalent vaccine, and (D) absence of pre-existing immunity (intramuscular PBS). n = 10 per group, except the vaccinated group receiving 4 µg CR9114, where n = 9. IM intramuscular, IN intranasal.