Figure 2.
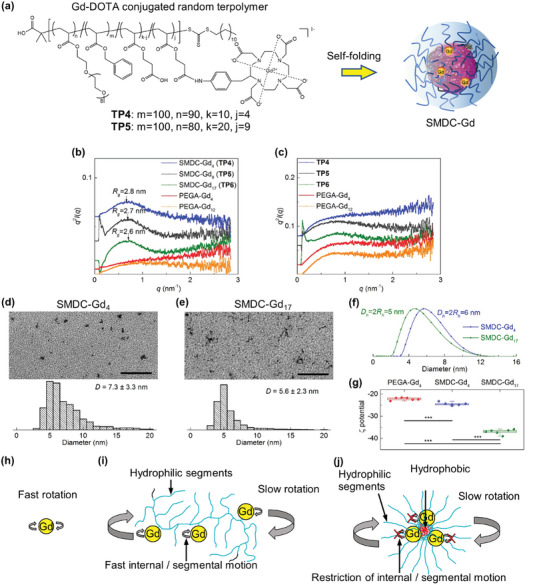
Characterizations of SMDC‐Gds. a) Chemical structure of Gd‐conjugated random terpolymers (TP4‐TP6), and schematic illustration of the SMDC‐Gd formation. b,c) SAXS Kratky plots of Gd‐loaded copolymers (TP4‐TP6, and PEGA‐Gds) in water or DMF at 25 °C: [copolymer] = 10 mg mL−1. SAXS plots in water showed the formation of SMDC‐Gds by TP4‐TP6. Plots in DMF demonstrated the reverse micelle formation for TP5‐TP6 and polymer chain conditions (no particle) for TP4 and PEGA‐Gds. d,e) TEM images and diameter distributions of SMDC‐Gd4 and SMDC‐Gd17 at 25 °C. TEM samples were prepared from the SMDC‐Gd aqueous solution from the aqueous solutions (10 mg mL−1) on a formvar/carbon‐supported copper grid. Scale bars, 100 nm. f) Size distributions of SMDC‐Gds determined by DLS at 25 °C: [copolymer] = 10 mg mL−1. g) ζ potentials of SMDC‐Gd4, SMDC‐Gd17, and PEGA‐Gd4 measured by Zetasizer at 25 °C: [copolymer] = 10 mg mL−1. Data are shown as box plot, n = 6, ⁎⁎⁎ p < 0.001. h–j) Strategies for increasing the relaxivity. Small molecular Gd complexes performed fast rotation and thus had relatively low relaxivity. Polymer conjugated Gd complexes exhibited slow rotation, but the relaxivity gain was limited by fast internal or segmental motion. SMDC conjugated Gd complexes produced a crowded complex environment, which enabled slow rotation while restricting internal or segmental motion, thus achieved higher relaxivities.
