Abstract
Lateral Meningocele or Lehman Syndrome (LMS) is associated with NOTCH3 mutations causing deletions of the PEST domain and a gain-of-NOTCH3 function. We demonstrated that Notch3em1Ecan mice harboring Notch3 mutations analogous to those found in LMS are osteopenic because of enhanced bone resorption. To determine the contribution of specific cell lineages to the phenotype, we created a conditional-by-inversion (Notch3COIN) model termed Notch3em2Ecan in which Cre recombination generates a Notch3INV allele expressing a NOTCH3 mutant lacking the PEST domain. Germline Notch3COIN inversion caused osteopenia and phenocopied the Notch3em1Ecan mutant, validating the model. To induce the mutation in osteocytes, smooth muscle and endothelial cells, Notch3COIN mice were bred with mice expressing Cre from the Dmp1, Sm22a and Cdh5 promoters, respectively, creating experimental mice harboring Notch3INV alleles in Cre-expressing cells and control littermates harboring Notch3COIN alleles. Notch3COIN inversion in osteocytes led to femoral and vertebral cancellous bone osteopenia, whereas Notch3COIN inversion in mural Sm22a or endothelial Cdh5-expressing cells did not result in a skeletal phenotype. In conclusion, introduction of the LMS mutation in osteocytes but not in vascular cells causes osteopenia and phenocopies Notch3em1Ecan global mutant mice.
Keywords: Mouse genetics, Notch pathway, Notch3, Lateral Meningocele Syndrome, Lehman Syndrome, Osteocyte, Osteopenia
1. INTRODUCTION
Notch receptors determine cell fate and function in a variety of tissues and organs including bone [1–3]. There are four Notch receptors, NOTCH 1–4, that are activated following interactions with ligands of the Jagged and Delta-like families, present in adjacent cells [1].
There is a degree of structural and functional overlap among the four Notch receptors. However, each Notch receptor has unique functions and specific patterns of cellular expression [3]. Notch1, 2 and 3 and low levels of Notch 4 transcripts are detected in skeletal cells. Whereas, Notch1 and Notch2 transcripts are present in osteoblasts and osteoclasts, Notch3 mRNA is detected in osteoblasts and osteocytes but not in the myeloid/osteoclast lineage [3–5]. NOTCH1 and NOTCH2 suppress osteoblast differentiation but NOTCH1 inhibits osteoclastogenesis whereas NOTCH2 enhances osteoclast differentiation [4, 6–9]. In contrast, NOTCH3 induces osteoclastogenesis by indirect mechanisms since it is not expressed in the myeloid lineage [10]. Previous work suggests that NOTCH3 acts by increasing the expression of receptor activator of nuclear factor-κB ligand (RANKL) in osteoblasts and osteocytes, and RANKL is required for osteoclastogenesis [10–13].
Lateral Meningocele Syndrome (LMS) or Lehman Syndrome (Online Mendelian Inheritance in Man 130720) is a rare genetic disorder characterized by craniofacial and skeletal abnormalities, meningoceles and neuromuscular dysfunction [14–16]. LMS is associated with short deletions or non-sense mutations in exon 33 of NOTCH3 that cause an early termination of translation and result in a NOTCH3 protein product that lacks the proline (P), glutamic acid (E), serine (S) and threonine (T) (PEST) domain [17]. Since the PEST domain is required for the degradation of NOTCH3, it is believed that its absence results in the stabilization of the NOTCH3 protein and as a consequence a gain-of-NOTCH3 function [17]. This is possible since there is a degree of ligand independent activation of NOTCH3 [1, 10, 18–20].
In an effort to understand the mechanisms responsible for LMS, we created a mouse model reproducing the functional aspects of the mutations found in subjects affected by the disease [10]. In this model, termed Notch3em1Ecan or Notch3tm1.1Ecan, a tandem termination codon was introduced into exon 33 of Notch3 causing the translation of a truncated NOTCH3 protein lacking the PEST domain. Notch3em1Ecan mice exhibit osteopenia due to an increase in osteoclast number despite the fact that Notch3 mRNA is not detected in the myeloid/osteoclast lineage [10]. We attributed the enhanced osteoclastogenesis to an induction of RANKL by cells of the osteoblast lineage, particularly osteocytes since they are a critical source of RANKL and as such influence bone remodeling [21, 22]. The cell responsible for the LMS phenotype could not be established with certainty since Notch3em1Ecan are global mutant mice. Whereas the osteocyte may be the cell responsible, Notch3 is expressed by mural vascular cells and NOTCH3 expressed in smooth muscle cells and vessels present in the bone microenvironment could influence the skeletal phenotype of LMS [23–25].
In the present study, a conditional by inversion (COIN) approach was applied to create a conditional mouse model of LMS (Notch3COIN or Notch3em2Ecan) [26, 27]. The model was designed to introduce a premature STOP codon into exon 33 of Notch3 following Cre-mediated recombination leading to the translation of a truncated NOTCH3 protein lacking the PEST domain and mimicking the genetic defect associated with LMS. To study the consequences of the NOTCH3 truncation in osteocytes, smooth muscle and endothelial cells, Notch3COIN mice were crossed with transgenics expressing Cre recombinase under the control of the dentin matrix protein 1 (Dmp1), the smooth muscle protein 22 alpha (Sm22a) or the VE Cadherin (Cdh5) promoter, respectively [28–30]. Mutant and control mice were examined for skeletal phenotypic changes by microcomputed tomography (μCT).
2. MATERIALS AND METHODS
2.1. Creation of the Notch3COIN Mouse Model
To generate a conditional allele of Notch3 modeling the LMS mutation, we used a Notch3 COIN vector. This vector consists of rabbit β-globin (Rbg) intron 2 sequence with a COIN module introduced into exon 33 of Notch3 in the anti-sense orientation immediately upstream of the PEST domain at nucleotide 6667 splitting exon 33 into two exons, with 4.5 kilobase (kb) 5’ and 1.5 kb 3’ homology arms, (Figure 1). In the sense orientation the COIN module is comprised of a gene cassette with lox66 upstream an Rbg splice acceptor, human influenza hemagglutinin (HA) coding sequence with a termination codon and an GTX internal ribosome entry site and enhanced green fluorescent protein (eGFP), Rbg polyA and a lox71 in an opposite orientation as that of lox66 [26, 31]. Cre recombination results in the inversion of the COIN sequence placing it in the sense orientation with HA in frame with Notch3 and resulting in a Notch3 allele encoding a bicistronic message comprised of Notch3ΔPEST-HA, 2223 amino acids of NOTCH3 tagged with HA and lacking the PEST domain. Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology was used to create a double-strand DNA break in Notch3 exon 33 and introduce the Notch3COIN between nucleotide 6666 and 6667 of Notch3 by homology-directed repair. The database http://crispor.tefor.net was used to scan Notch3 sequences to identify high score target sites for potential single guide (sg) RNA. Notch3 sgRNA 5’- CTA CTG CGG GTT CCT GCT GC was selected because of its high score and limited probabilities of off-target effects and was designed for Cas9 to cleave exon 33 of Notch3 adjacent to a protospacer adjacent motif (PAM) positioned at nucleotide 6664 to 6666. The DNA donor and Notch3 sgRNA/Cas9 ribonucleoprotein (RNP) complex were co-injected directly into the pronucleus of C57BL/6J one-cell embryos [32–34]. Injected embryos were transferred into CD1 pseudo-pregnant foster females, and potential founders were screened for the presence of the COIN module by polymerase chain reaction (PCR) and positive pups were screened for proper targeting by nested long-range PCR for founder identification. Founders were bred with wild type C57BL/6J mice and F1 pups were screened by nested long-range PCR to confirm their identity. Two live founders were generated and crossed with C57BL/6J mice to establish Notch3COIN (syn Notch3em2Ecan) lines.
Figure 1. Engineering of the Notch3COIN allele.
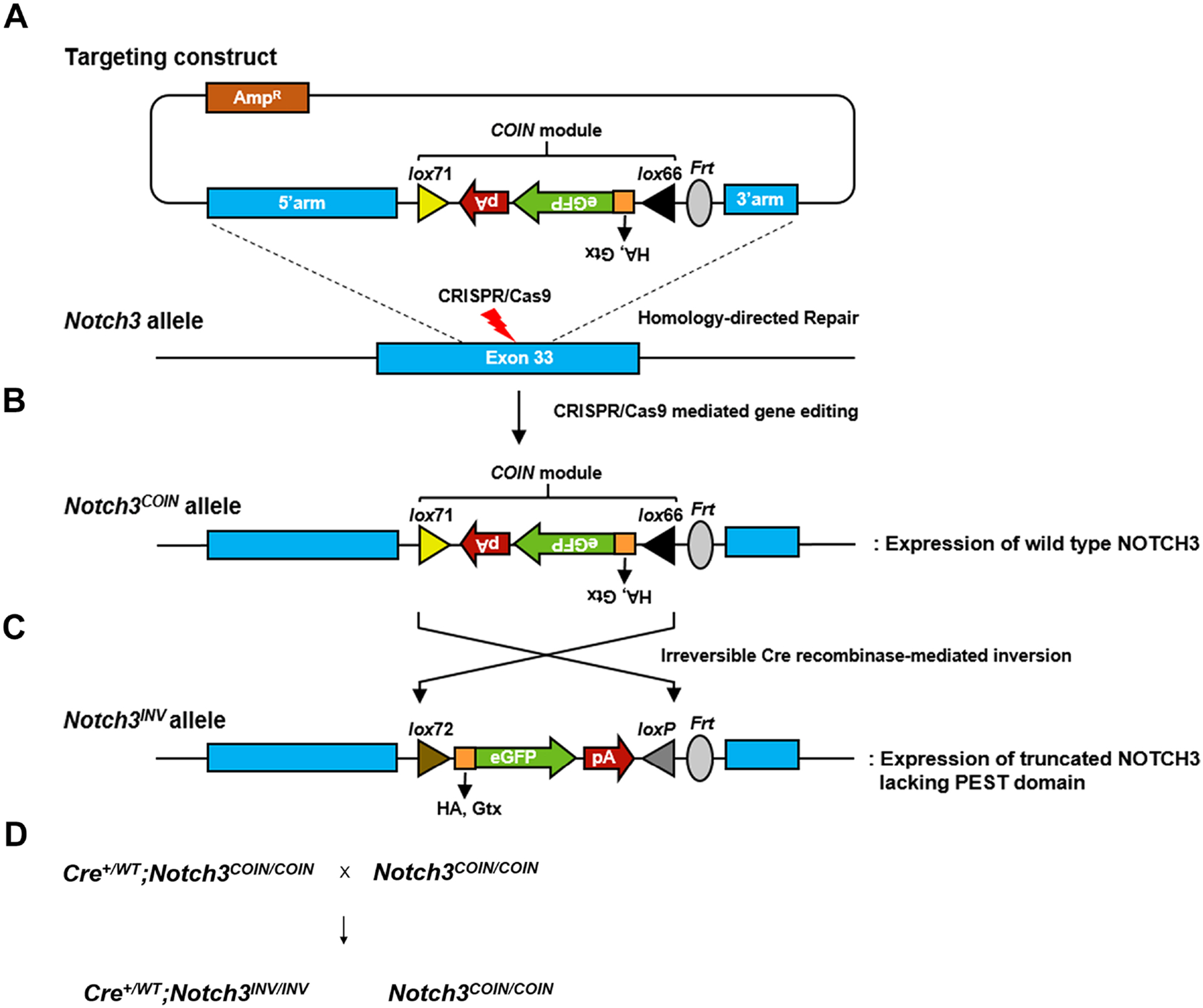
A. Representation of the targeting construct and silent COIN module in the anti-sense orientation consisting of lox71, rabbit β-globin (Rbg) polyadenylation signal (pA), enhanced green fluorescence protein (eGFP)-coding sequence, Gtx internal ribosome entry site, human influenza hemagglutinin (HA) tag coding sequence, 3’-splice region from the second intron of the Rbg gene (not shown) and lox66. B. Representation of CRISPR-Cas9 gene editing, and C. Generation of the Notch3INV allele by Cre recombinase-mediated inversion of the COIN module, and maturation of the Notch3INV transcript, which is translated into a NOTCH3 mutant protein lacking the PEST domain. D. Mating scheme
2.2. Induction of the LMS Mutation in the Germ line, Osteocytes and Vascular Cells
To achieve the systemic inversion of the Notch3COIN allele, F1 heterozygous Notch3COIN/WT (Notch3em2Ecan) male mice were bred with female mice expressing Cre under the control of the Hprt promoter (HprtCre) (Hprttm1(CAG-cre)Mnn/J, Jackson Laboratory 004302) [35]. This resulted in the germ line inversion of the COIN module and consequent creation of mice heterozygous for the Notch3INV allele and termed Notch3em2.1Ecan. The latter were crossed with wild type C57BL/6J mice to generate Notch3INV/WT experimental and wild type controls for study. To induce inversion of the COIN module in osteocytes, C57BL/6J mice harboring a transgene, where the Cre recombinase is cloned downstream of a 9.6 kb murine Dmp1 promoter fragment (Dmp1-Cre) (Tg(Dmp1-cre)1Jqfe/BwdJ, Jackson 023047) were used [28]. To invert the COIN allele in smooth muscle cells, C57BL/6J mice harboring a transgene where the Cre recombinase coding sequence is driven by a 2.8 kb of murine Sm22a or transgelin sequences (Sm22a-Cre or Tagln-Cre, Jackson Laboratory, B6.Cg-Tg(Tagln-cre)1Her/J, 017491) were used [29]. To induce the inversion of the COIN allele in endothelial cells, C57BL/6J mice harboring a transgene where the Cre recombinase coding sequence is driven by a 2.5 kb fragment of the murine VE Cadherin (Cdh5)-Cre, Jackson Laboratory, B6.FVB-Tg(Cdh5-cre)7Mlia/J, 006137) promoter were used [30].
Hemizygous Dmp1, Sm22a or Cdh5 Cre transgenics homozygous for the Notch3COIN allele (Cre+/−;Notch3COIN/COIN) were crossed with Notch3COIN/COIN mice to generate Cre+/−;Notch3INV/INV and Notch3COIN/COIN littermate controls (Figure 1). Allelic composition was determined by PCR analysis of tail DNA with specific primers, and inversion of the COIN module was documented by PCR analysis in DNA from tails (Notch3em2.1Ecan), tibiae or aorta (Notch3em2Ecan) (all primers were from Integrated DNA Technologies (IDT), Coralville, IA; Table 1).
Table 1.
Primers used for allele identification.
| Allele | Strand | Sequence | Amplicon Size (bp) |
|---|---|---|---|
| Genotyping | |||
| Cdh5-Cre | Forward | 5’-GCGGTCTGGCAGTAAAAACTATC -3’ | 100 |
| Dmp1-Cre | Forward | 5’-CCCGCAGAACCTGAAGATG-3’ | 534 |
| Hprt WT | Forward | 5’-TTTCTATAGGACTGAAAGACTTGCTC-3’ | 200 |
| Hprt Cre | Forward | 5’-GCGGTCTGGCAGTAAAAACTATC-3’ | 100 |
| Notch3 | Forward | 5’-GAGGCCCAAGGAATCGAGAC-3’ | 269 |
| Notch3 COIN | Forward | 5’-CCGGGCCGCGACTGAAACCCTAG-3’ | 158 |
| Sm22a-Cre | Forward | 5’-GCGGTCTGGCAGTAAAAACTATC -3’ | 100 |
| LoxP Recombination | |||
| Notch3 INV | Forward | 5’- CTGCTCCAGGACATGGAGAGG -3’ | Non rec no band |
Studies were approved by the Institutional Animal Care and Use Committee of UConn Health.
2.3. Microcomputed Tomography (μCT)
Femoral microarchitecture was determined using a μCT instrument (Scanco μCT 40, Scanco Medical AG, Bassersdorf, Switzerland), which was calibrated at periodic intervals with a manufacturer provided phantom [36, 37]. Femurs and vertebrae from control and experimental mice were scanned in 70% ethanol at high resolution, energy level of 55 peak kilovoltage (kVp), intensity of 145 μA, and integration time of 200 ms as reported [7, 10]. For cancellous microarchitecture, 160 slices at the distal femoral metaphysis or ~500 slices of L3 were acquired at an isotropic voxel size of 216 μm3 and a slice thickness of 6 μm and chosen for analysis. Cancellous bone volume fraction (bone volume/total volume) and microarchitecture were evaluated starting ~1.0 mm proximal from the femoral condyles. For L3, the vertebral body was scanned in its entirety. Contours were manually drawn every 10 slices, a few voxels away from the endocortical boundary, to define the region of interest for analysis, whereas the remaining slice contours were iterated automatically. Total volume, bone volume, bone volume fraction, trabecular thickness, trabecular number, connectivity density, structure model index (SMI), and material density were measured in trabecular regions using a Gaussian filter (σ = 0.8) and defined thresholds. A threshold of 240 permil equivalent to 355.5 mg of hydroxyapatite was used [36, 37]. For analysis of cortical bone, contours were iterated across 100 slices along the cortical shell of the femoral midshaft, excluding the marrow cavity. Analysis of bone volume/total volume, porosity, cortical thickness, total cross-sectional and cortical bone area, segmented bone area, periosteal and endosteal perimeter, and material density were conducted using a Gaussian filter (σ = 0.8, support = 1) with thresholds of 390 or 400 permil equivalent to 682.9 or 704.7mg of hydroxyapatite/cm3, respectively.
2.4. Bone Histomorphometry
For cancellous bone histomorphometric analysis, femurs were dissected, fixed in 70% ethanol and embedded in methyl methacrylate. Femurs were sectioned at a thickness of 5 μm along the sagittal plane on a Microm microtome (Richards-Allan Scientific, Kalamazoo, MI), and stained with 0.1% toluidine blue. Parameters of bone morphometry were measured in a defined area between 0.35 mm and 2.16 mm from the growth plate at a magnification of 100x using an OsteoMeasure morphometry system (Osteometrics, Atlanta, GA). Stained sections were used to draw bone tissue and to measure trabecular separation, number and thickness, osteoid and eroded surface, as well as to count osteoblast and osteoclast number [38]
2.5. Osteocyte-enriched Cell Preparations
Osteocyte-enriched cells were obtained from Dmp1-Cre;Notch3INV/INV and Notch3COIN/COIN control mice following a modification of a previously described method [5, 39]. Femurs or tibiae were removed aseptically from experimental and control mice, dissected free from surrounding tissues, the proximal epiphyseal end was excised, and the bone marrow was removed by centrifugation. The distal epiphysis was excised, and bones were digested for 20 min at 37 °C with type II bacterial collagenase pretreated with N-α-tosyl-l-lysyl-chloromethyl ketone hydrochloride and subsequently exposed to 5 mM EDTA for 20 min at 37 °C. The resulting osteocyte-enriched cortical bones from experimental and control mice were cultured for 72 hours in Dulbecco Modified Medium (DMEM) supplemented with 10% fetal bovine serum prior to RNA extraction [5, 40].
2.6. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted with the micro RNeasy kit (Qiagen, Valencia, CA), in accordance with manufacturer’s instructions, as previously reported [7, 10, 41, 42]. Equal amounts of RNA were reverse transcribed using the iScript RT-PCR kit (Bio-Rad) and amplified in the presence of specific primers (IDT) (Table 2) with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) at 60 °C for 35 cycles. Transcript copy number was estimated by comparison with a serial dilution of cDNA for Alpl (encoding for alkaline phosphatase from American Type Tissue Culture Collection (ATCC), Manassas, VA), Bglap (encoding for osteocalcin from J. Lian, University of Vermont), Sost (from Thermo Fisher Scientific, Waltham, MA), Tnfsf11 (encoding for RANKL from Source BioScience, Nottingham, UK), Tnfrsf11b (encoding for osteoprotegerin from ATCC), or Notch3PEST (U. Lendahl, Addgene #47618, Watertown, MA) [43]. Notch3INV or Notch3ΔPEST copy number was estimated by comparison to a serial dilution of ~100 base pair synthetic DNA fragment (IDT) containing HA sequences that are only detectable in the inverted allele, and cloned into pcDNA3.1 (−) (Thermo Fisher Scientific, Waltham, MA). Amplification reactions were conducted in a CFX96 qRT-PCR detection system (Bio-Rad), and fluorescence was monitored during every PCR cycle at the annealing step. Data are expressed as copy number corrected for Rpl38 (from ATCC) [44].
Table 2.
Primers used for qRT-PCR determinations. GenBank accession numbers identify transcript recognized by primer pairs.
| Gene | Strand | Sequence | GenBank Accession Number |
|---|---|---|---|
| Alpl | Forward | 5’-TGGTATGGGCGTCTCCACAGTAACC-3’ | NM_007431 |
| Bglap | Forward | 5’-GACTCCGGCGCTACCTTGGGTAAG-3’ | NM_001037939 |
| Notch3 PEST * | Forward | 5’-CATCCTTATTTGACCCCGTC-3’ | NM_008716 |
| Notch3 ΔPEST(HA tag) ** | Forward | 5’-AGTTTCTCCCCAAGAGCG-3’ | Not applicable |
| Rpl38 | Forward | 5’-AGAACAAGGATAATGTGAAGTTCAAGGTTC-3’ |
NM_001048057; NM_001048058; |
| Sost | Forward | 5’-AGGAATGATGCCACAGAGGTC-3’ | NM_024449 |
| Tnfrsf11b | Forward | 5′-CAGAAAGGAAATGCAACACATGACAAC-3′ | NM_008764 |
| Tnfsf11 | Forward | 5’-TATAGAATCCTGAGACTCCATGAAAAC-3’ | NM_011613 |
recognizes a fragment coding for the PEST domain of NOTCH3 so that it amplifies the product from wild type and non-inverted (floxed) allele, but not from inverted alleles.
recognizes a fragment coding for the HA tag of the truncated NOTCH3 so that it amplifies only the product from an inverted allele.
2.7. Statistics
Data are expressed as means ± SD. All data represent biological replicates. qRT-PCR values were derived from two technical replicates of biological replicates as indicated in the text and legends. Statistical differences were determined by unpaired Student’s t-test for pairwise comparisons or by two-way analysis of variance for multiple comparison with Holm-Šidák post-hoc analysis using GraphPad Prism version 9.3.1 for Mac OS, GraphPad Software (San Diego, CA).
3. RESULTS
3.1. Generation of a Conditional LMS Mouse Model
To introduce the LMS mutation into selected cell populations, Notch3COIN mice were created by inserting an artificial COIN intron into exon 33 of the murine Notch3 locus and were termed Notch3em2Ecan (Figure 1). Prior to Cre recombination the COIN module, a gene trap-like lox66_HA-egfp-polyA_lox71 cassette placed in the antisense orientation, is removed by splicing of the precursor mRNA to generate a Notch3COIN transcript that is indistinguishable from the Notch3WT mRNA (Figure 1). In the presence of the Cre recombinase, which recognizes the lox71 and lox66 mutant sites in a mirror image configuration, the lox66_HA-egfp-polyA_lox71 cassette is brought into the sense strand, causing the irreversible conversion of the COIN allele. The resulting allele encodes for a message that is translated into an HA-tagged NOTCH3 mutant lacking the PEST domain (Figure 1). This allele was termed Notch3INV. The proper integration of the COIN module in the Notch3em2Ecan mouse model was verified by sequencing of PCR products of the 5’ and 3’ arms of the vector used in founder and F1 mice. In addition, heterozygous crosses of Notch3em2Ecan mice generated a homozygous offspring documenting loss of wild type alleles.
3.2. Inversion of the Notch3COIN Allele in the Germ line Causes Osteopenia
To validate the Notch3em2Ecan (Notch3COIN) mouse as a model of LMS, the skeletal phenotype of Notch3INV mice created by inversion of the COIN module in the germ line was determined. To this end, Notch3COIN/WT male mice were crossed with Hprt-Cre female mice to create Notch3INV/WT mice, and these were termed Notch3em2.1Ecan. Heterozygous Notch3em2.1Ecan mice were crossed with wild type mice to create Notch3em2.1Ecan heterozygous and control wild type littermates for study. COIN inversion was documented by the presence of the Notch3INV allele in DNA from tails of Notch3em2.1Ecan mice, and qRT-PCR analysis of total RNA from calvariae confirmed the expression of the Notch3ΔPEST transcript, expressing the HA tag, in mutant mice but not in control littermates (Figure 2).
Figure 2. Inversion of the Notch3COIN allele in the germ line causes osteopenia.
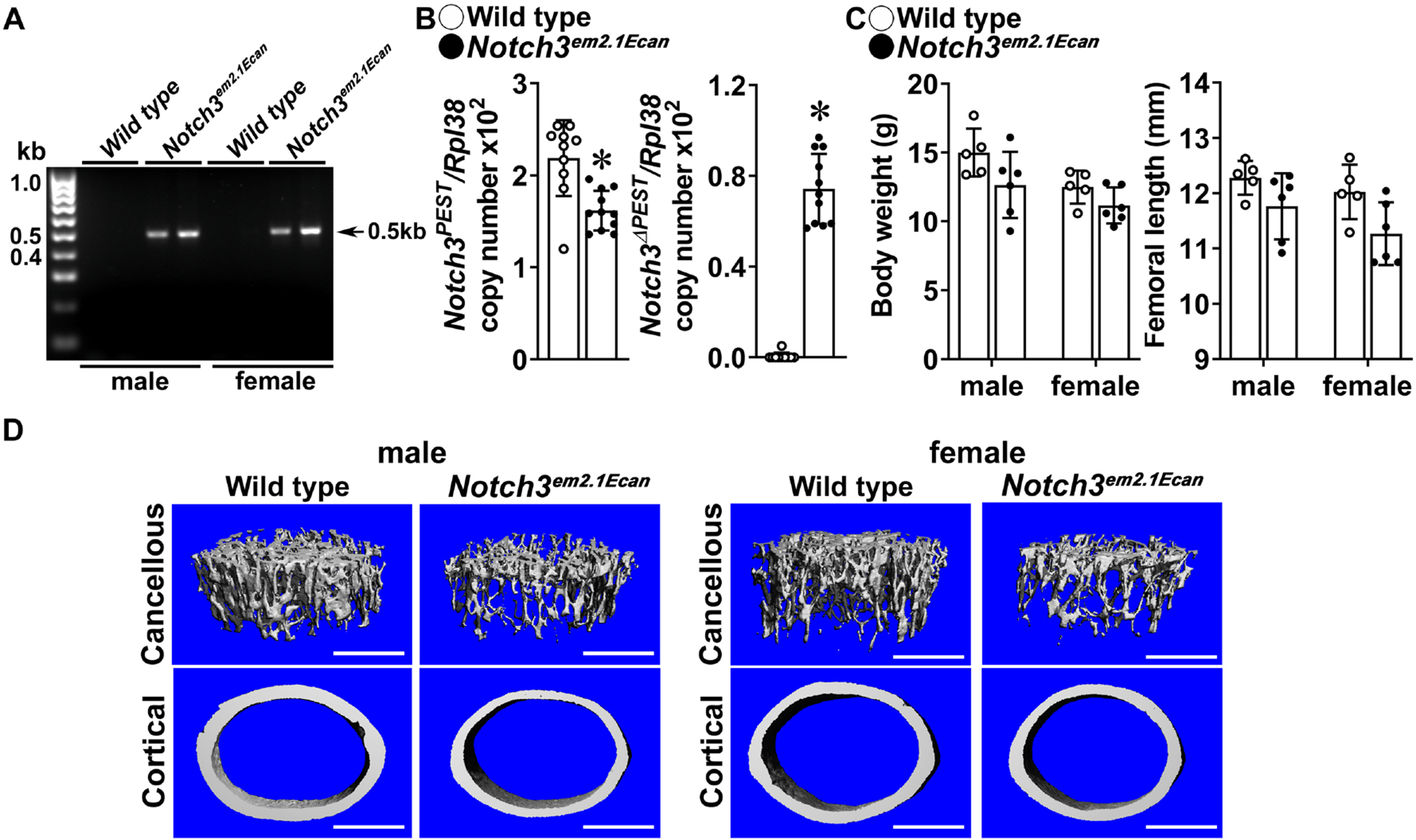
A. DNA was extracted from tail of heterozygous Notch3em2.1Ecan (Hprt-Cre;Notch3INV/WT) and wild type littermate controls and Notch3COIN inversion was documented by gel electrophoresis of PCR products obtained with primers specific for the Notch3INV allele. Arrows indicate the position of the 495 base pair (bp) amplicon. B. Total RNA was extracted from calvariae of 1 month old Notch3em2.1Ecan mutants (closed circles) and wild type littermate controls (open circles), and expression of the Notch3 PEST and Notch3ΔPEST mRNA measured by qRT-PCR. Transcript levels are reported as copy number corrected for Rpl38 mRNA levels. Bars represent means and ranges S.D.; n = 9 for control, n = 11 for Notch3em2.1Ecan, all biological replicates. C. Weight in gm and femoral length in mm of 1 month old Notch3em2.1Ecan (closed circles) and wild type control littermates (open circles). Bars represent means and ranges SD; n = 5 for control males and females, n = 6 for Notch3em2.1Ecan males and females. D. Representative μCT images of femoral proximal trabecular bone and midshaft cortical bone of 1 month old control and Notch3em2.1Ecan mice. The scale bar in the right corner represents 500 μm. Complete data set in Table 3. *Significantly different between control and Notch3em2.1Ecan, p < 0.05 by unpaired t-test for Panel B and ANOVA for Panel C.
One month old heterozygous Notch3em2.1Ecan male and female mice appeared normal, albeit a small not significant reduction in weight was observed in male and femoral length was noted in female mice. μCT analysis of the distal femur revealed a 45% decrease in trabecular bone volume/total volume in 1 month old Notch3em2.1Ecan mice of both sexes (Table3, Figure 2). The decrease was secondary to a reduced number of trabeculae and to a lesser extent to a decrease in trabecular thickness. Connectivity density was lower and SMI was higher in Notch3em2.1Ecan mice than in controls, indicating a prevalence of rod-like trabeculae (Table 3, Figure 2). Notch3em2.1Ecan mice had a decrease in cortical bone area and a non-significant decrease in periosteal perimeter suggesting smaller bones. Bone histomorphometry of 1 month old Notch3em2.1Ecan mice confirmed the decrease in bone volume and trabecular number, but did not reveal changes in osteoblast number, osteoclast number or eroded surface (Table 4). The results are consistent with the phenotype reported for global Notch3LMS mutant (Notch3em1Ecan) mice, and validate the Notch3COIN mouse as a model to study the contribution of selected cell lineages to the phenotype [10].
Table 3.
Femoral microarchitecture assessed by μCT of 1 month old Notch3em2.1Ecan (Hprt-Cre;Notch3INV/WT) mice and sex-matched wild type littermates (Control).
| Males | Females | |||
|---|---|---|---|---|
| Control | Notch3 INV | Control | Notch3 INV | |
| n = 5 | n = 6 | n = 5 | n = 6 | |
| Distal Femur Trabecular Bone | ||||
| Bone Volume/Total Volume (%) | 12.5 ± 2.5 | 6.7 ± 1.8* | 9.0 ± 1.2 | 5.0 ± 0.7* |
| Trabecular Separation (μm) | 195 ± 10 | 251 ± 30* | 216 ± 14 | 288 ± 16* |
| Trabecular Number (1/mm) | 5.3 ± 0.2 | 4.1 ± 0.5* | 4.7 ± 0.3 | 3.5 ± 0.2* |
| Trabecular Thickness (μm) | 35 ± 3 | 31 ± 3* | 31 ± 1 | 29 ± 1 |
| Connectivity Density (1/mm3) | 411 ± 77 | 223 ± 54* | 299 ± 60 | 169 ± 29* |
| Structure Model Index | 1.9 ± 0.3 | 2.5 ± 0.1* | 2.2 ± 1.1 | 2.5 ± 0.1* |
| Density of Material (mg HA/cm3) | 775 ± 8 | 753 ± 12* | 776 ± 7 | 764 ± 8 |
| Femoral Midshaft Cortical Bone | ||||
| Bone Volume/Total Volume (%) | 82.0 ± 1.4 | 80.3 ± 2.5 | 82.4 ± 0.7 | 81.2 ± 0.9 |
| Porosity (%) | 18.0 ± 1.4 | 19.7 ± 2.5 | 17.6 ± 0.7 | 18.8 ± 0.9 |
| Cortical Thickness (μm) | 88 ± 5 | 82 ± 9 | 86 ± 2 | 80 ± 4 |
| Total Area (mm2) | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Bone Area (mm2) | 0.49 ± 0.03 | 0.42 ± 0.05* | 0.45 ± 0.02 | 0.41 ± 0.03 |
| Marrow Area (mm2) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Periosteal Perimeter (μm) | 4.4 ± 0.1 | 4.2 ± 0.2 | 4.3 ± 0.1 | 4.1 ± 0.2 |
| Endocortical Perimeter (mm) | 3.6 ± 0.3 | 3.5 ± 0.2 | 3.6 ± 0.1 | 3.5 ± 0.2 |
| Density of Material (mg HA/cm3) | 960 ± 20 | 946 ± 23 | 974 ± 10 | 962 ± 11 |
μCT was performed at the femoral distal end for trabecular or midshaft for cortical bone. Values are means ± S.D.
Significantly different between control and Notch3em2.1Ecan mice, p < 0.05 by ANOVA.
Table 4.
Cancellous bone histomorphometry of 1 month old Notch3em2.1Ecan (Hprt-Cre;Notch3INV/WT) male mice and sex-matched wild type littermates (controls).
| Males | ||
|---|---|---|
| Cancellous Bone | Control n = 5 | Notch3INV n = 4 |
| Static Histomorphometry | ||
| Bone Volume/Tissue Volume (%) | 16.3 ± 2.8 | 9.6 ± 5.0* |
| Trabecular Separation (μm) | 195 ± 26 | 355 ± 152* |
| Trabecular Number (1/mm) | 4.3 ± 0.4 | 2.8 ± 0.9* |
| Trabecular Thickness (μm) | 38 ± 5 | 32 ± 1 |
| Osteoblast Surface/Bone Surface (%) | 21.2 ± 4.8 | 21.6 ± 8.6 |
| Osteoblasts/Bone Perimeter (1/mm) | 16.8 ± 4.6 | 17.4 ± 6.3 |
| Osteoid Surface/Bone Surface (%) | 1.7 ± 0.9 | 1.2 ± 0.9 |
| Osteoclast Surface/Bone Surface (%) | 9.7 ± 2.5 | 9.8 ± 4.7 |
| Osteoclasts/Bone Perimeter (1/mm) | 3.6 ± 0.9 | 3.6 ± 1.8 |
| Eroded Surface/Bone Surface (%) | 2.3 ± 0.8 | 2.0 ± 1.5 |
Histomorphometry was carried out on sagittal sections of distal femurs. Values are means ± S.D.
Significantly different between control and Notch3em2.1Ecan mice, p < 0.05 by unpaired t-test.
3.3. Inversion of the Notch3COIN Allele in Osteocytes Causes Osteopenia
Because Notch3 is preferentially expressed by osteocytes, we wished to determine whether the osteopenia observed in Notch3em1Ecan LMS global mutant mice was driven by an effect in cells of the osteocytic lineage [3, 5]. For this purpose, Dmp1-Cre;Notch3COIN/COIN (Notch3em2Ecan) were crossed with Notch3COIN/COIN mice to create Dmp1-Cre;Notch3INV/INV mice and littermate Notch3COIN/COIN controls. Inversion of the COIN allele was detected in DNA from tibiae of Dmp1-Cre;Notch3INV/INV mice but not in littermate controls (Figure 3). Accordingly, the Notch3ΔPEST transcript was detected only in calvariae from Dmp1-Cre;Notch3INV/INV mice, documenting the induction of the mutation in cells that express Dmp1.
Figure 3. Inversion of the Notch3COIN allele in osteocytes causes osteopenia.
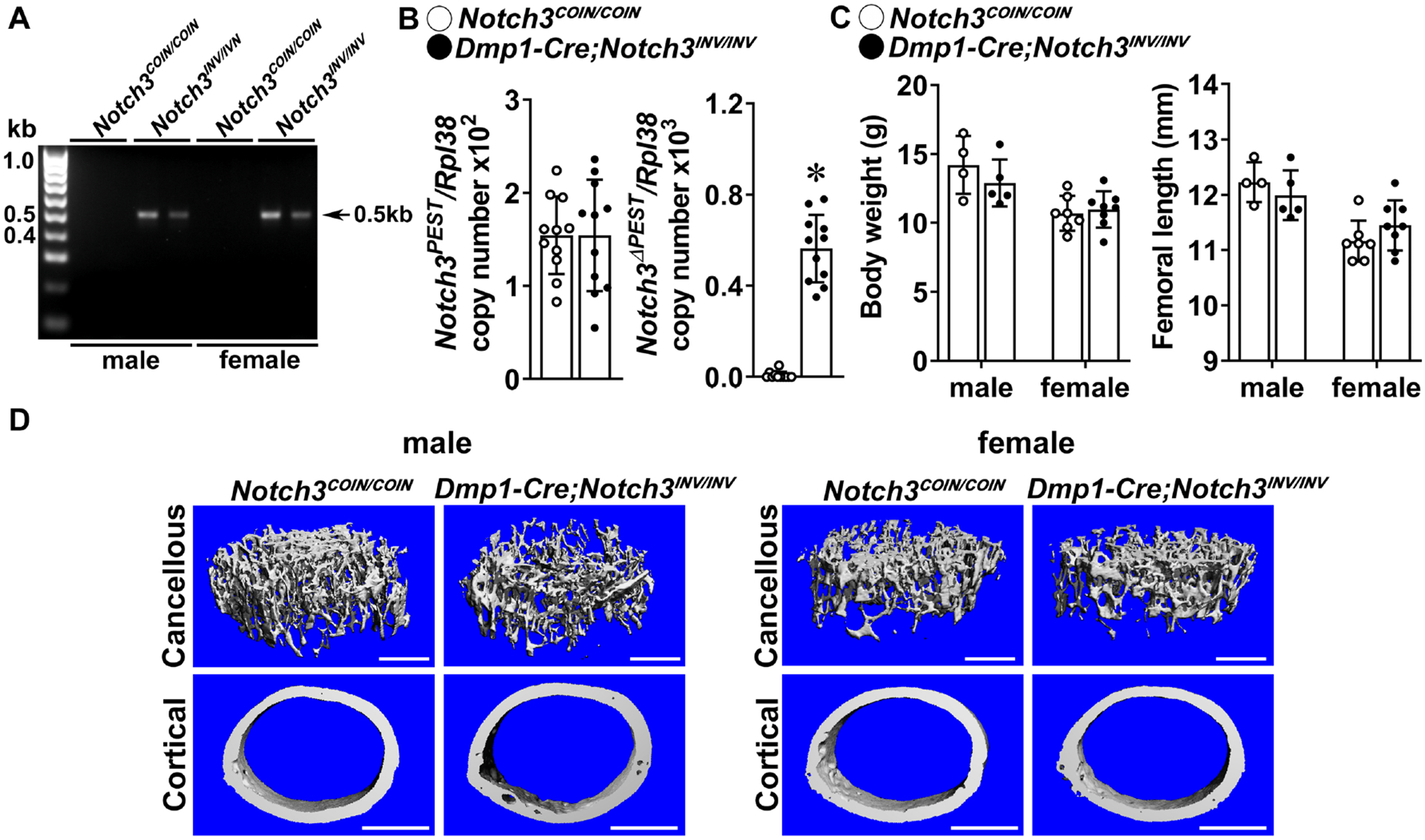
A. DNA was extracted from tibiae and Notch3COIN inversion was documented by gel electrophoresis of PCR products obtained with primers specific for the Notch3INV allele. Arrow indicates the position of the 495 bp amplicon. B. Total RNA was extracted from calvariae, and expression of the Notch3PEST and Notch3ΔPEST mRNA measured by qRT-PCR of 1 month old Dmp1-Cre;Notch3INV/INV mutants (closed circles) and Notch3COIN/COIN littermate controls (open circles). Transcript levels are reported as copy number corrected for Rpl38 levels. Bars represent means and ranges S.D.; n = 4 for control, n = 4 for Dmp1-Cre;Notch3INV/INV, all biological replicates. C. Weight in gm and femoral length in mm of 1 month old Dmp1-Cre;Notch3INV/INV (closed circles) and Notch3COIN/COIN littermate controls (open circles). Bars represent means and ranges SD; n = 4 males and n = 7 females for control, and n = 5 males and n = 8 females for Notch3INV/INV. D. Representative μCT images of femoral proximal trabecular bone and midshaft cortical bone of 1 month old control Notch3COIN/COIN and Dmp1-Cre;Notch3INV/INV male and female mice. The scale bar in the right corner represents 500 μm. Complete data set in Table 5. *Significantly different between control Notch3COIN/COIN and Dmp1-Cre;Notch3INV/INV, p < 0.05 by unpaired t-test.
The general appearance, weight and femoral length of 1 month old Dmp1-Cre;Notch3INV/INV mice were not different from those of control sex-matched littermates (Figure 3). At 1 month of age, μCT revealed significant cancellous bone osteopenia in Dmp1-Cre;Notch3INV/INV male mice; this was associated with decreased connectivity density and increased SMI (both p < 0.05 by unpaired t-test; p > 0.05 by ANOVA), indicating a prevalence of rod-like over plate-like trabeculae (Table 5, Figure 3). Cortical bone and female mice were not affected. The cancellous bone osteopenic phenotype was confirmed in vertebral (L3) bone of male 1 month old Dmp1-Cre;Notch3INV/INV mice. Bone volume/total volume was decreased from (means ± SD; n = 4 to 5) 13.0 ± 0.6 in control Notch3COIN/COIN to 11.1 ±0.4 in Dmp1-Cre;Notch3INV/INV mice (p < 0.05 by unpaired t-test; p > 0.05 by ANOVA). Bone histomorphometric analysis of 1 month old male Dmp1-Cre;Notch3INV mice did not reveal changes in osteoblast or osteoclast number compared to control sex-matched Notch3COIN mice (data not shown).
Table 5.
Femoral microarchitecture assessed by μCT of 1 month old Dmp1-Cre;Notch3INV/INV mice and sex-matched Notch3em2Ecan (Notch3COIN/COIN) littermate controls.
| Males | Females | |||
|---|---|---|---|---|
| Notch3 COIN | Notch3 INV | Notch3 COIN | Notch3 INV | |
| n = 4 | n = 5 | n = 7 | n = 8 | |
| Distal Femur Trabecular Bone | ||||
| Bone Volume/Total Volume (%) | 10.0 ± 1.2 | 7.6 ± 1.3* | 6.2 ± 1.3 | 5.8 ± 1.3 |
| Trabecular Separation (μm) | 200 ± 10 | 222 ± 19 | 268 ± 33 | 279 ± 24 |
| Trabecular Number (1/mm) | 5.0 ± 0.3 | 4.6 ± 0.4 | 3.8 ± 0.5 | 3.7 ± 0.3 |
| Trabecular Thickness (μm) | 31 ± 0 | 30 ± 1 | 30 ± 1 | 29 ± 2 |
| Connectivity Density (1/mm3) | 329 ± 52 | 230 ± 55 | 197 ± 70 | 195 ± 42 |
| Structure Model Index | 2.1 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.3 |
| Density of Material (mg HA/cm3) | 767 ± 17 | 761 ± 10 | 751 ± 12 | 741 ± 20 |
| Femoral Midshaft Cortical Bone | ||||
| Bone Volume/Total Volume (%) | 83.4 ± 1.4 | 82.7 ± 1.3 | 81.1 ± 1.2 | 80.2 ± 2.4 |
| Porosity (%) | 16.6 ± 1.4 | 17.3 ± 1.3 | 18.9 ± 1.2 | 19.8 ± 2.4 |
| Cortical Thickness (μm) | 91 ± 6 | 87 ± 5 | 81 ± 4 | 80 ± 6 |
| Total Area (mm2) | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.2 |
| Bone Area (mm2) | 0.47 ± 0.04 | 0.44 ± 0.05 | 0.41 ± 0.03 | 0.41 ± 0.05 |
| Marrow Area (mm2) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 |
| Periosteal Perimeter (μm) | 4.3 ± 0.2 | 4.2 ± 0.2 | 4.1 ± 0.2 | 4.2 ± 0.3 |
| Endocortical Perimeter (mm) | 3.6 ± 0.2 | 3.5 ± 0.1 | 3.5 ± 0.1 | 3.5 ± 0.3 |
| Density of Material (mg HA/cm3) | 959 ± 27 | 956 ± 27 | 929 ± 15 | 917 ± 3 |
μCT was performed at the femoral distal end for trabecular or midshaft for cortical bone. Values are means ± S.D.
Significantly different between control and Notch3INV/INV mice, p < 0.05 by unpaired ANOVA.
3.4. Inversion of the Notch3COIN Allele Induces Tnfsf11 but does not Change Alpl, Bglap or Sost Expression
To determine possible mechanisms responsible for the skeletal phenotype of the Dmp1-Cre;Notch3INV/INV mice, osteocyte-enriched preparations were obtained from tibiae of Dmp1-Cre;Notch3INV/INV and control Notch3COIN/COIN littermates. Tnfsf11 (encoding RANKL) was induced in osteocytes from Dmp1-Cre;Notch3INV/INV mice, whereas the expression of Tnfrsf11b (encoding osteoprotegerin) was not changed (Figure 4).
Figure 4. Inversion of the Notch3COIN allele in osteocytes induces RANKL.
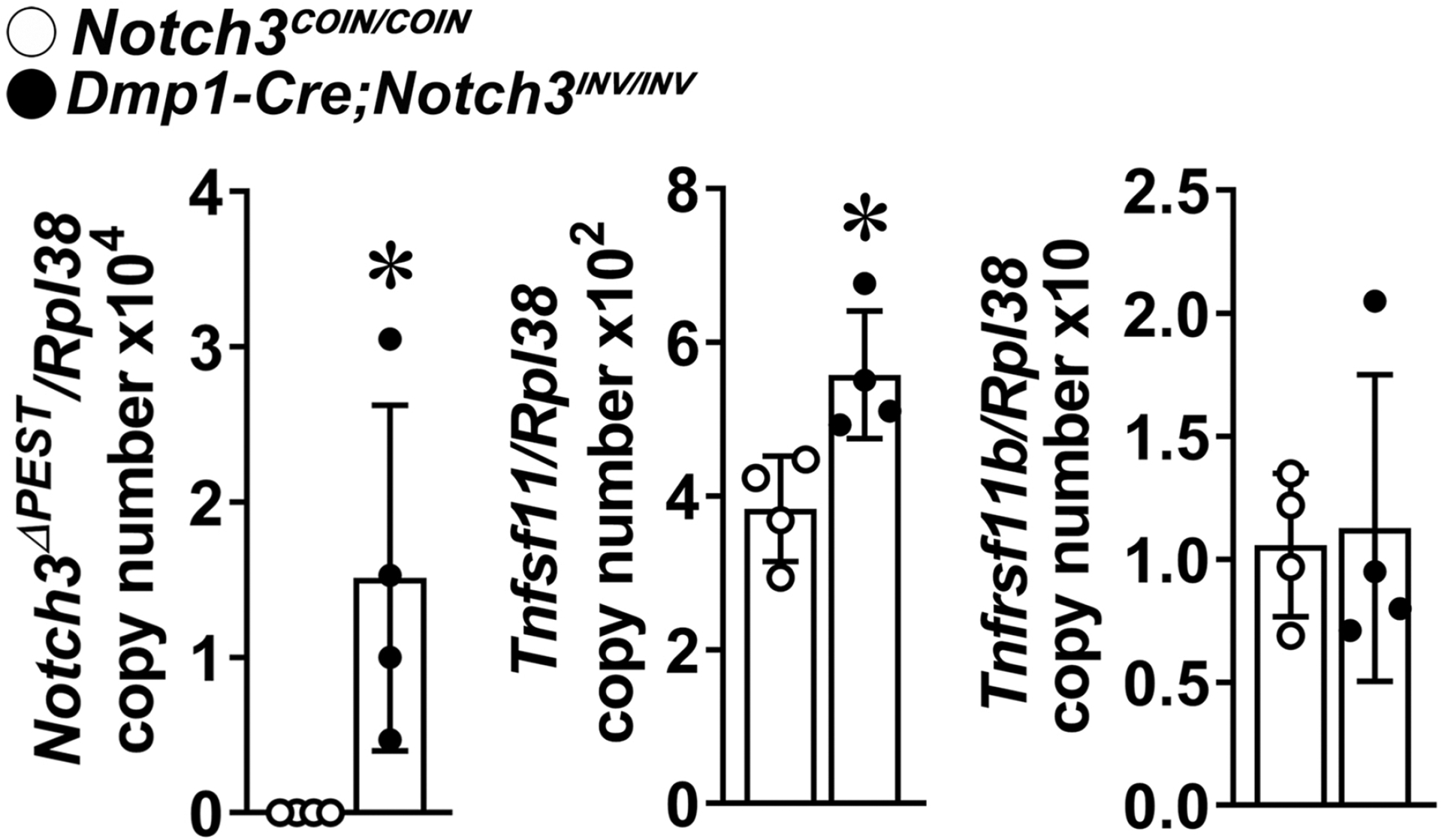
Total RNA isolated from osteocyte-enriched cells from Dmp1-Cre;NotchINV/INV (closed circles) and Notch3COIN/COIN control (open circles) mice was extracted, and gene expression was determined by qRT-PCR. Data are expressed as Notch3ΔPEST, Tnfsf11 (RANKL) and Tnfrsf11b (osteoprotegerin) copy number corrected for Rpl38. Bars represent means and ranges SD; n = 4. Data are derived from biological replicates. *Significantly different between control Notch3COIN/COIN and Dmp1-Cre;Notch3INV/INV, p < 0.05 by unpaired t-test.
To ascertain whether there were possible changes in osteoblast/osteocyte function in Notch3INV mice, calvariae from Notch32.1Ecan (germline inverted) and Dmp1-Cre;Notch3INV/INV were extracted and examined for gene expression. There were no significant differences between control and experimental mice in Alpl, Bglap or Sost mRNA levels (all values copy number/Rpl38; means ± SD; n = 10 – 11). Alpl 2.1 ± 0.2 in wild type and 1.6 ± 0.2 in Notch32.1Ecan mice, Bglap 11.9 ± 1.2 in wild type and 9.5 ± 0.8 in Notch32.1Ecan mice (both p > 0.05), and Sost 2.8 ± 0.3 in wild type and 2.0 ± 0.2 in Notch32.1Ecan mice (p < 0.053). Alpl 1.2 ± 0.1 in control and 1.3 ± 0.1 in Dmp1-Cre;NotchINV/INV mice, Bglap 6.4 ± 0.9 in control and 7.7 ± 1.8 in Dmp1-Cre;Notch3INV/INV, and Sost 2.2 ± 0.3 in control and 1.9 ± 0.3 in Dmp1-Cre;Notch3INV/INV mice (all p > 0.05).
3.5. Inversion of the Notch3COINAllele in Vascular Cells does not Cause Osteopenia
Because NOTCH3 is the prevalent Notch receptor expressed in mural vascular cells, we asked whether the activation of the Notch3LMS mutation in vascular cells could be responsible for the osteopenic phenotype [24, 25, 45]. To this end, Sm22a-Cre;Notch3COIN/COIN mice were crossed with Notch3COIN/COIN to introduce the Notch3LMS mutation in smooth muscle cells. One month old Sm22a-Cre;Notch3INV/INV mice appeared normal, and their weight and femoral length were not different from control Notch3COIN/COIN mice (Figure 5). Inversion of the COIN allele was documented in DNA from the aorta of Sm22a-Cre;Notch3INV/INV mice and inversion did not occur in control mice. Accordingly, Notch3ΔPEST transcripts were detected in aorta from Sm22a-Cre;Notch3INV/INV mice. Although a modest degree of inversion of the COIN allele was detected in tibiae from Sm22a-Cre;Notch3INV/INV mice, there was no induction of Notch3ΔPEST transcript in this tissue indicating that the mutant transcript was not expressed in the bone environment. Accordingly, cancellous and cortical bone architecture revealed no differences between 1 month old Sm22a-Cre;Notch3INV/INV mice and control littermates (Table 6).
Figure 5. Inversion of the Notch3COIN allele in smooth muscle cells does not cause osteopenia.
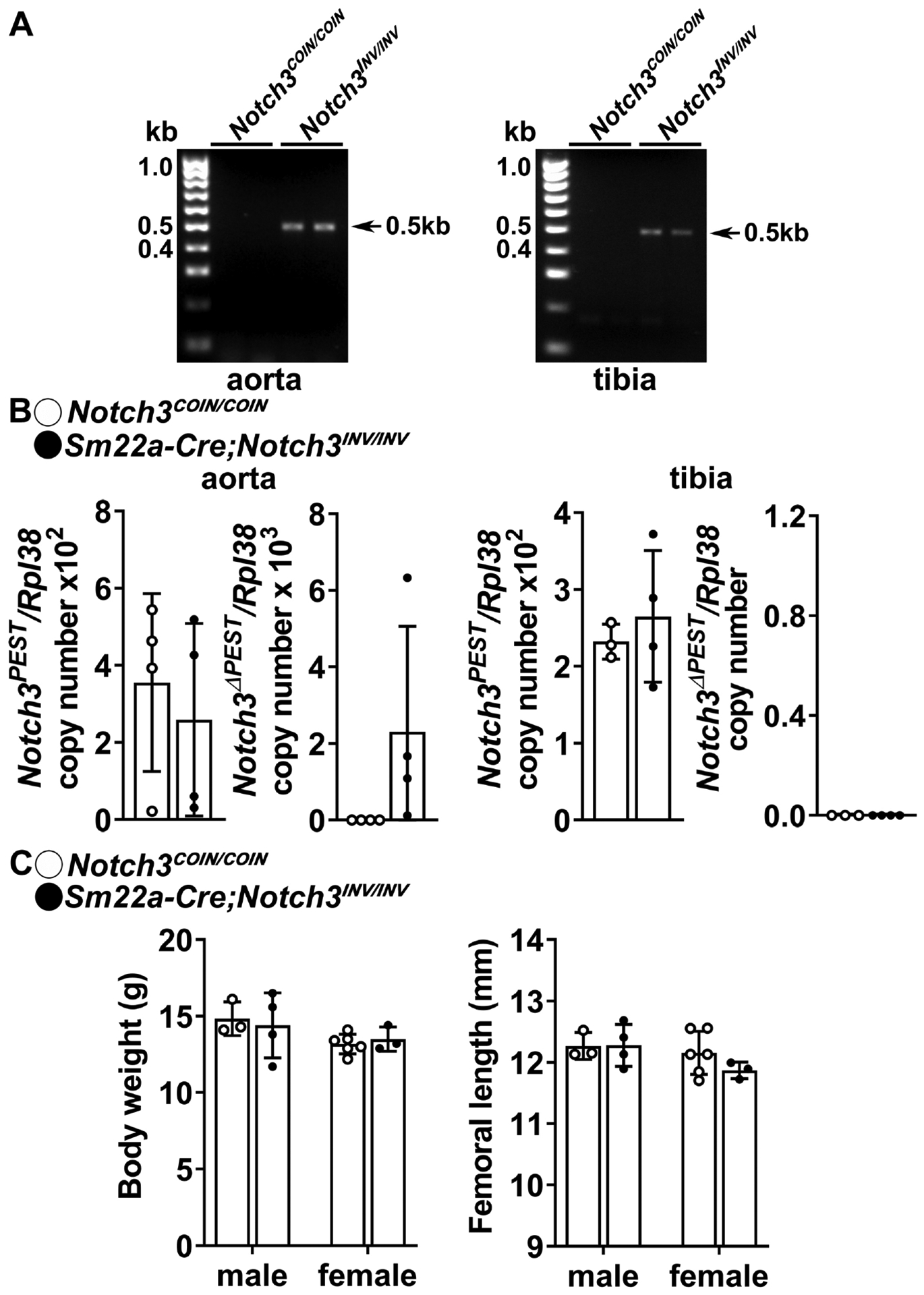
A. DNA was extracted from aorta and tibiae, and Notch3COIN inversion was documented by gel electrophoresis of PCR products obtained with primers specific for the Notch3INV allele. Arrows indicate the position of the 495 bp amplicon. B. Total RNA was extracted from aorta and tibiae, and expression of the Notch3PEST and Notch3ΔPEST mRNA measured by qRT-PCR in 1 month old Sm22a-Cre;Notch3INV/INV mutants (closed circles) and Notch3COIN/COIN littermate controls (open circles). Transcript levels are reported as copy number corrected for Rpl38 levels. Bars represent means and ranges S.D.; n = 3–4 for control and n = 4 for Sm22a-Cre;Notch3INV/INV, all biological replicates. C. Weight in gm and femoral length in mm of 1 month old Sm22a-Cre;Notch3INV/INV (closed circles) and Notch3COIN/COIN littermates (open circles). Bars represent means and ranges SD; n = 3 males and n = 6 females for control and n = 4 males and n = 3 females for Sm22a-Cre;Notch3INV.
Table 6.
Femoral microarchitecture assessed by μCT of 1 month old Sm22a-Cre;Notch3INV/INV mice and Notch3em2Ecan (Notch3COIN/COIN) sex-matched littermate controls.
| Males | Females | |||
|---|---|---|---|---|
| Notch3 COIN | Notch3 INV | Notch3 COIN | Notch3 INV | |
| n = 3 | n = 4 | n = 6 | n = 3 | |
| Distal Femur Trabecular Bone | ||||
| Bone Volume/Total Volume (%) | 6.9 ± 1.1 | 5.9 ± 1.7 | 4.7 ± 0.8 | 4.5 ± 0.4 |
| Trabecular Separation (μm) | 252 ± 31 | 274 ± 28 | 320 ± 32 | 316 ± 36 |
| Trabecular Number (1/mm) | 4.0 ± 0.4 | 3.7 ± 0.2 | 3.2 ± 0.3 | 3.3 ± 0.3 |
| Trabecular Thickness (μm) | 33 ± 2 | 33 ± 2 | 33 ± 2 | 32 ± 1 |
| Connectivity Density (1/mm3) | 155 ± 44 | 117 ± 33 | 95 ± 21 | 94 ± 33 |
| Structure Model Index | 2.4 ± 0.1 | 2.6 ± 0.2 | 2.6 ± 0.1 | 2.6 ± 0.1 |
| Density of Material (mg HA/cm3) | 784 ± 7 | 778 ± 13 | 762 ± 15 | 774 ± 4 |
| Femoral Midshaft Cortical Bone | ||||
| Bone Volume/Total Volume (%) | 82.6 ± 1.5 | 83.2 ± 1.1 | 82.3 ± 1.5 | 82.9 ± 1.2 |
| Porosity (%) | 17.5 ± 1.5 | 16.8 ± 1.1 | 17.7 ± 1.5 | 17.1 ± 1.2 |
| Cortical Thickness (μm) | 89 ± 2 | 95 ± 4 | 90 ± 15 | 93 ± 6 |
| Total Area (mm2) | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Bone Area (mm2) | 0.50 ± 0.03 | 0.50 ± 0.04 | 0.50 ± 0.01 | 0.51 ± 0.02 |
| Marrow Area (mm2) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Periosteal Perimeter (μm) | 4.4 ± 0.1 | 4.3 ± 0.2 | 4.4 ± 0.1 | 4.5 ± 0.1 |
| Endocortical Perimeter (mm) | 3.7 ± 0.1 | 3.5 ± 0.2 | 3.6 ± 0.1 | 3.7 ± 0.1 |
| Density of Material (mg HA/cm3) | 964 ± 20 | 972 ± 18 | 960 ± 21 | 969 ± 9 |
μCT was performed at the femoral distal end for trabecular or midshaft for cortical bone. Values are means ± S.D.
Although Notch3 is not expressed or expressed at very low levels in endothelial cells, Notch activation in endothelial cells can influence bone remodeling and NOTCH3 activity in mural cells [23, 25]. To determine whether the introduction of the Notch3LMS mutation in endothelial cells caused or did not cause a skeletal phenotype, Cdh5-Cre;Notch3COIN/COIN mice were crossed with Notch3COIN/COIN to induce Notch3 COIN inversion in endothelial cells. One month old Cdh5-Cre;Notch3INV/INV mice appeared normal, and their weight and femoral length were not substantially different from controls (Figure 6). Cre-mediated inversion of the COIN allele was documented in aorta and tibiae, and low levels of Notch3ΔPEST mRNA were detected in tibiae but not in aorta from Cdh5-Cre;Notch3INV/INV mice. Copy number revealed that the expression of Notch3ΔPEST transcripts was 1/100 the one observed in bones from Notch3em2.1Ecan (germline inverted) mice and Dmp1-Cre;Notch3INV/INV mice. The modest induction of Notch3ΔPEST in tibiae was possibly due to the presence of small vessels in the bone environment, and bone microarchitecture was not different between 1 month old Cdh5-Cre;Notch3INV/INV and Notch3em2Ecan control littermate mice (Table 7).
Figure 6. Inversion of the Notch3COIN allele in endothelial cells does not cause osteopenia.
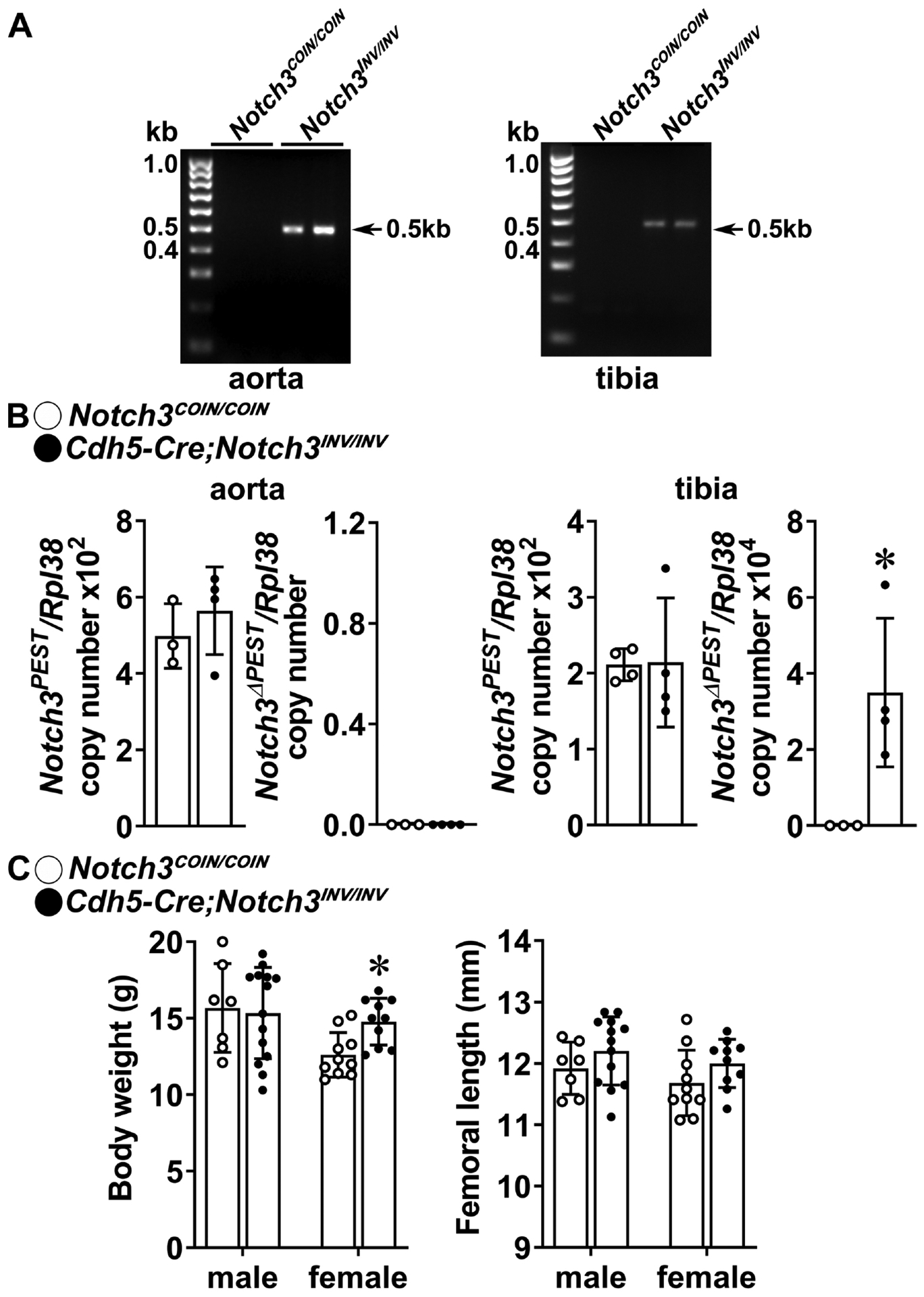
A. DNA was extracted from aorta and tibiae and Notch3COIN inversion was documented by gel electrophoresis of PCR products obtained with primers specific for the Notch3INV allele. Arrows indicate the position of the 495 bp amplicon. B. Total RNA was extracted from aorta and tibiae, and expression of the Notch3PEST and Notch3ΔPEST mRNA measured by qRT-PCR in 1 month old Cdh5-Cre;Notch3INV/INV mutants (closed circles) and Notch3COIN/COIN littermate controls (open circles). Transcript levels are reported as copy number corrected for Rpl38 mRNA levels. Bars represent means and ranges S.D.; n = 3–4 for control and n = 4 Cdh5-Cre;Notch3INV/INV, all biological replicates. C. Weight in gm and femoral length in mm of 1 month old Cdh5-Cre;Notch3INV/INV (closed circles) and Notch3COIN/COIN littermates (open circles). Bars represent means and ranges SD; n = 7 males and n = 10 females for control and n = 14 males and n = 10 females for Cdh5-Cre;Notch3INV/INV. *Significantly different between control Notch3COIN/COIN and Cdh5-Cre;Notch3INV/INV, p < 0.05 by unpaired t-test.
Table 7.
Femoral microarchitecture assessed by μCT of 1 month old Cdh5-Cre;Notch3INV/INV mice and Notch3em2Ecan (Notch3COIN/COIN) sex-matched littermate controls.
| Males | Females | |||
|---|---|---|---|---|
| Notch3 COIN | Notch3 INV | Notch3 COIN | Notch3 INV | |
| n = 7 | n = 14 | n = 10 | n = 10 | |
| Distal Femur Trabecular Bone | ||||
| Bone Volume/Total Volume (%) | 7.5 ± 1.7 | 8.1 ± 1.7 | 5.1 ± 0.1 | 5.7 ± 0.8 |
| Trabecular Separation (μm) | 250 ± 20 | 241 ± 18 | 289 ± 31 | 284 ± 33 |
| Trabecular Number (1/mm) | 4.1 ± 0.3 | 4.2 ± 0.3 | 3.5 ± 0.4 | 3.6 ± 0.4 |
| Trabecular Thickness (μm) | 34 ± 5 | 35 ± 4 | 30 ± 2 | 32 ± 1 |
| Connectivity Density (1/mm3) | 176 ± 28 | 199 ± 42 | 140 ± 53 | 136 ± 31 |
| Structure Model Index | 2.4 ± 0.2 | 2.3 ± 0.2 | 2.5 ± 0.1 | 2.4 ± 0.1 |
| Density of Material (mg HA/cm3) | 765 ± 23 | 778 ± 18 | 763 ± 16 | 766 ± 14 |
| Femoral Midshaft Cortical Bone | ||||
| Bone Volume/Total Volume (%) | 81.4 ± 2.3 | 82.3 ± 2.0 | 82.0 ± 1.4 | 82.8 ± 1.3 |
| Porosity (%) | 18.6 ± 2.3 | 17.7 ± 2.0 | 18.0 ± 1.4 | 17.2 ± 1.3 |
| Cortical Thickness (μm) | 89 ± 8 | 91 ± 10 | 87 ± 5 | 91 ± 5 |
| Total Area (mm2) | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Bone Area (mm2) | 0.50 ± 0.07 | 0.51 ± 0.08 | 0.44 ± 0.03 | 0.49 ± 0.03 |
| Marrow Area (mm2) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Periosteal Perimeter (μm) | 4.3 ± 0.2 | 4.4 ± 0.2 | 4.2 ± 0.1 | 4.3 ± 0.2 |
| Endocortical Perimeter (mm) | 4.3 ± 0.2 | 3.6 ± 0.2 | 3.4 ± 0.1 | 3.6 ± 0.2 |
| Density of Material (mg HA/cm3) | 949 ± 34 | 961 ± 22 | 957 ± 23 | 965 ± 18 |
μCT was performed at the femoral distal end for trabecular or midshaft for cortical bone. Values are means ± S.D.
Significantly different between control and Notch3INV/INV mice by unpaired ANOVA.
4. DISCUSSION
In previous studies, we have shown that a mouse model harboring a Notch3 mutation reproducing the functional outcomes of LMS exhibits osteopenia [10]. However, the cell responsible for the phenotype was not identified because the model used harbors the Notch3 mutation in all cell lineages. The establishment of the cell responsible for the phenotype is of particular interest in the case of NOTCH3 since its transcript expression is not detected in the myeloid/osteoclast lineage [3]. Instead, Notch3 is mostly expressed in osteocytes and mural vascular cells [3, 5, 24]. The present study, aimed at establishing the contributions of specific cell lineages to the LMS skeletal phenotype. This was possible by the creation of a COIN mouse model termed Notch3em2Ecan.
The pathogenic variants associated with LMS occur within exon 33 of NOTCH3, and the conditional insertion of a premature STOP codon in the homologous region of the murine Notch3 locus was achieved by the creation of a COIN allele. The COIN module was introduced without disrupting the expression or function of the targeted allele under basal conditions, and this would not be possible with traditional Cre-loxP approaches [26]. Notch3em2.1Ecan mutants of both sexes, generated by germ line inversion of the COIN module, exhibited cancellous and cortical bone osteopenia phenocopying Notch3em1Ecan global LMS mutants and validating the Notch3COIN approach.
Selective introduction of the LMS mutation in osteocytes led to osteopenia, albeit this was modest and restricted to male mutant mice since female mice were not affected. Although Dmp1 is preferentially expressed by osteocytes, Dmp1 promoter activity is also detected in mature osteoblasts, so that the osteopenic phenotype observed is secondary to an effect in both cells [46, 47]. The bone loss occurred mostly at femoral sites. Although an increased expression of RANKL without a concomitant alteration in osteoprotegerin expression was found in osteocytes from Dmp1-Cre;Notch3INV/INV mice, bone histomorphometry did not reveal an increase in osteoclast number. Osteocyte-derived RANKL plays a pivotal role in bone remodeling, but other mechanisms could have played a role in the phenotype [21, 48, 49]. Since Dmp1 is expressed in mineralized tissues during development, it is possible that the phenotype of Dmp1-Cre;Notch3INV/INV was due to effects of NOTCH3 during skeletal development [50]. It is important to mention that mice of both sexes displayed osteopenia in the global Notch3em1Ecan mouse model, and following the germline inversion of Notch3em2Ecan conditional mutants. There is no apparent explanation for the sex dimorphism of the Dmp1-Cre;Notch3INV/INV skeletal phenotype, and there was no evidence of less efficient Cre recombination in osteocytes from female than from male mice. This dimorphism was also noted when exploring the effects of the Notch ligand Delta-like 4 (DLL4) in bone, and female mice were more resistant to the actions of DLL4 and this was attributed to their lower bone surface [51]. The sexual dimorphism also could be related to a higher rate of bone remodeling in young female than in male mice, a characteristic of the C57BL/6J genetic background [37]. It is important to note that the number of osteocytes is not substantially different in bones from male and female young C57BL/6J mice, so that the expression of Dmp1 would be expected to be similar in both sexes [40]. Estrogens induce the Notch ligand JAGGED 1 (JAG1) and NOTCH1 in breast cancer MCF7 endothelial cells and bone marrow stromal cells and as a consequence activate Notch signaling [52, 53]. This would suggest that the lack of a phenotype in female mice is not related to an estrogen effect, which would be expected to amplify the Notch signal. Evidence of an estrogen effect on Notch signaling in vivo is less compelling [54].
Although the skeletal phenotype of Dmp1-Cre;Notch3INV/INV male mice mirrors the phenotype of global Notch3em1Ecan mice and of germ line inverted Notch3em2.1Ecan mice, it is of a more modest nature. This may relate to insufficient recombination in osteocytes/osteoblasts or may indicate that other cells harboring the Notch3 LMS mutation contribute to the osteopenic phenotype.
In contrast to the direct actions of NOTCH1 and NOTCH2 on osteoclast cell differentiation, NOTCH3 has distinct and indirect effects in the myeloid lineage, since it is not expressed in these cells [3, 10]. NOTCH3 induces osteoclastogenesis only by indirect mechanisms and previous work in cells from global Notchem1Ecan mice demonstrated enhanced osteoclastogenesis in co-cultures of bone marrow derived macrophages from wild type mice with osteoblasts from Notch3em1Ecan mice. This suggested that the cell responsible for the Notch3em1Ecan phenotype was of the osteoblast lineage, possibly by increasing the expression of RANKL. This could have important therapeutic implications in individuals with LMS and bone loss, which could be ameliorated by the administration of anti-RANKL antibodies, such a denosumab. This has been the case for the related disorder, Hajdu Cheney Syndrome, which is associated with NOTCH2 mutations and a NOTCH2 gain of function [55, 56].
The skeleton contains various types of mural and endothelial cells that play an important role in osteogenesis, hematopoiesis and vascular homeostasis and endothelial cells are the most important secretory cells in the bone environment [57–59]. Activation of Notch signaling in endothelial cells promotes angiogenesis and osteogenesis, whereas the inactivation of Rbpjk or Dll4, and consequent decline in Notch canonical signaling in endothelial cells results in impaired angiogenesis and bone loss [59]. However, the induction of the Notch3LMS mutation in Cdh5-expressing cells did not result in a skeletal phenotype. This is most likely related to the low level or no expression of Notch3 in endothelial cells [60]. Notch3 is expressed in vascular smooth muscle cells where it plays a critical role in blood vessel wall integrity [60–62]. The induction of the Notch3LMS mutation in Sm22a smooth muscle-expressing cells did not cause a skeletal phenotype [63]. However, Notch3ΔPEST transcripts were not detected in tibiae of Sm22a-Cre;Notch3INV/INV mice possibly because the expression of Sm22a is limited to mural vascular cells not present in the bone environment and explaining the absence of the skeletal phenotype. This does not exclude a role of NOTCH3 in the structure of mural vessels, it simply indicates that smooth muscle vascular NOTCH3 does influence bone remodeling.
Limitations of this work include: 1) The efficiency of the various Cre drivers used in the bone environment was not equal and could have contributed to differences in the phenotype. To assess Cre-dependent activity, we determined Notch3ΔPEST transcripts expressed only after recombination. Notch3ΔPEST was not detected in bones from Sm22a-Cre;Notch3INV/INV mice and copy number in Cdh5-Cre;Notch3INV/INV was about 100 times lower than in bone from Dmp1-Cre;Notch3INV/INV mice indicating different levels of Cre recombination efficiency, at least in bone, possibly explaining the different phenotypes; 2) Cre;Notch3INV/INV mice were compared to Cre negative Notch3COIN/COIN littermates and no additional controls, such as wild type mice were utilized for comparison; 3) The phenotype was established in young mice prior to the attainment of skeletal maturity and the phenotype could be secondary to developmental events and might not persist at maturity; and 4) The mechanisms responsible for the osteopenia of Hprt-Cre;Notch3INV/WT and Dmp1-Cre;Notch3INV/INV mice were not elucidated.
5. CONCLUSIONS
In conclusion, expression of a Notch3 mutant lacking the PEST domain and mimicking LMS in osteocytes causes osteopenia in male mice.
Highlights.
Lateral Meningocele Syndrome (LMS) is associated with NOTCH3mutations
A conditional by inversion (COIN) model was used to introduce Notch3 LMS mutations
Germline Notch3COIN inversion resulted in osteopenia
Introduction of the Notch3 LMS mutation in osteocytes caused osteopenia
Introduction of the Notch3 LMS mutation in vascular cells did no cause osteopenia
ACKNOWLEDGMENTS
The authors thank Magda Mocarska for technical assistance and Mary Yurczak for secretarial support.
FUNDING
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases [AR072987] (EC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- bp
base pair
- cadh5
VE Cadherin
- CRISPR
clustered regularly interpersonal short palindromic repeats
- COIN
conditional by inversion
- Dmp1
dentin matrix protein 1
- DMEM
Dulbecco Modified Medium
- eGFP
enhanced green fluorescent protein
- kVp
kilovoltage
- HA
hemagglutinin
- kb
kilobase
- LMS
Lateral Meningocele Syndrome
- μCT
microcomputed tomography
- PCR
polymerase chain reaction
- pA
polyadenylation signal
- PEST
proline (P), glutamic acid (E), serine (S) and threonine (T) domain
- PAM
protospacer adjacent motif
- qRT-PCR
quantitative reverse transcript-polymerase chain reaction
- Rbg
rabbit β-globin
- RANKL
receptor activator of nuclear factor-κB ligand
- RNA-Seq
RNA sequencing
- RNP
ribonucleoprotein
- sg
single guide
- Sm22a
smooth muscle protein 22 alpha
- SMI
structure model index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
ANE is employed by Regeneron Pharmaceuticals. EC, SPY, LS and JY declare no conflicts of interest with the contents of this article.
CRediT Author Statement
Ernesto Canalis: Conceptualization, Validation, Formal Analysis, Resources, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project Administration, Funding Acquisition; Siu-Pok Yee: Methodology, Resources, Writing – Review & Editing; Aris N. Economides: Methodology, Resources, Writing – Review & Editing; Lauren Schilling: Investigation, Writing - Review & Editing; Jungeun Yu: Validation, Formal Analysis, Investigation, Data Curation, Writing - Review & Editing, Visualization
REFERENCES
- [1].Siebel C, Lendahl U, Notch Signaling in Development, Tissue Homeostasis, and Disease, Physiol. Rev 97(4) (2017) 1235–1294. 10.1152/physrev.00005.2017 [DOI] [PubMed] [Google Scholar]
- [2].Zanotti S, Canalis E, Notch Signaling and the Skeleton, Endocr. Rev 37(3) (2016) 223–253. 10.1210/er.2016-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Canalis E, Notch in skeletal physiology and disease, Osteoporos.Int 29(12) (2018) 2611–2621. 10.1007/s00198-018-4694-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL, NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells, J. Biol. Chem 283(10) (2008) 6509–6518. [DOI] [PubMed] [Google Scholar]
- [5].Zanotti S, Canalis E, Parathyroid hormone inhibits Notch signaling in osteoblasts and osteocytes, Bone 103 (2017) 159–167. 10.1016/j.bone.2017.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K, The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis, Mol. Cell Biol 28(20) (2008) 6402–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Canalis E, Schilling L, Yee SP, Lee SK, Zanotti S, Hajdu Cheney Mouse Mutants Exhibit Osteopenia, Increased Osteoclastogenesis and Bone Resorption, J. Biol. Chem 291 (2016) 1538–1551. 10.1074/jbc.M115.685453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Canalis E, Parker K, Feng JQ, Zanotti S, Osteoblast Lineage-specific Effects of Notch Activation in the Skeleton, Endocrinology 154(2) (2013) 623–634. en.2012–1732 [pii]; 10.1210/en.2012-1732 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yu J, Canalis E, The Hajdu Cheney mutation sensitizes mice to the osteolytic actions of tumor necrosis factor alpha, J. Biol. Chem 294(39) (2019) 14203–14214. 10.1074/jbc.RA119.009824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Canalis E, Yu J, Schilling L, Yee SP, Zanotti S, The lateral meningocele syndrome mutation causes marked osteopenia in mice, J. Biol. Chem 293(36) (2018) 14165–14177. 10.1074/jbc.RA118.004242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu J, Canalis E, Notch and the regulation of osteoclast differentiation and function, Bone 138 (2020) 115474. 10.1016/j.bone.2020.115474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T, Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts, Dev. Cell 3(6) (2002) 889–901. [DOI] [PubMed] [Google Scholar]
- [13].Tsukasaki M, Takayanagi H, Osteoimmunology: evolving concepts in bone-immune interactions in health and disease, Nat. Rev. Immunol 19(10) (2019) 626–642. 10.1038/s41577-019-0178-8 [DOI] [PubMed] [Google Scholar]
- [14].Lehman RA, Stears JC, Wesenberg RL, Nusbaum ED, Familial osteosclerosis with abnormalities of the nervous system and meninges, J. Pediatr 90(1) (1977) 49–54. [DOI] [PubMed] [Google Scholar]
- [15].Gripp KW, Scott CI Jr., Hughes HE, Wallerstein R, Nicholson L, States L, Bason LD, Kaplan P, Zderic SA, Duhaime AC, Miller F, Magnusson MR, Zackai EH, Lateral meningocele syndrome: three new patients and review of the literature, Am. J. Med. Genet 70(3) (1997) 229–239. [PubMed] [Google Scholar]
- [16].Avela K, Valanne L, Helenius I, Makitie O, Hajdu-Cheney syndrome with severe dural ectasia, Am. J. Med. Genet. A 155A(3) (2011) 595–598. 10.1002/ajmg.a.33510 [doi] [DOI] [PubMed] [Google Scholar]
- [17].Gripp KW, Robbins KM, Sobreira NL, Witmer PD, Bird LM, Avela K, Makitie O, Alves D, Hogue JS, Zackai EH, Doheny KF, Stabley DL, Sol-Church K, Truncating mutations in the last exon of NOTCH3 cause lateral meningocele syndrome, Am. J. Med. Genet. A 167A(2) (2015) 271–281. 10.1002/ajmg.a.36863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choy L, Hagenbeek TJ, Solon M, French D, Finkle D, Shelton A, Venook R, Brauer MJ, Siebel CW, Constitutive NOTCH3 Signaling Promotes the Growth of Basal Breast Cancers, Cancer Res. 77(6) (2017) 1439–1452. 10.1158/0008-5472.CAN-16-1022 [DOI] [PubMed] [Google Scholar]
- [19].Xu X, Choi SH, Hu T, Tiyanont K, Habets R, Groot AJ, Vooijs M, Aster JC, Chopra R, Fryer C, Blacklow SC, Insights into Autoregulation of Notch3 from Structural and Functional Studies of Its Negative Regulatory Region, Structure 23(7) (2015) 1227–1235. 10.1016/j.str.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tiyanont K, Wales TE, Siebel CW, Engen JR, Blacklow SC, Insights into Notch3 activation and inhibition mediated by antibodies directed against its negative regulatory region, J. Mol. Biol 425(17) (2013) 3192–3204. 10.1016/j.jmb.2013.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xiong J, O’Brien CA, Osteocyte RANKL: New insights into the control of bone remodeling, J. Bone Miner. Res 27(3) (2012) 499–505. 10.1002/jbmr.1547 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dallas SL, Prideaux M, Bonewald LF, The osteocyte: an endocrine cell … and more, Endocr. Rev 34(5) (2013) 658–690. 10.1210/er.2012-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ramasamy SK, Kusumbe AP, Wang L, Adams RH, Endothelial Notch activity promotes angiogenesis and osteogenesis in bone, Nature 507(7492) (2014) 376–380. 10.1038/nature13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A, Notch3 is required for arterial identity and maturation of vascular smooth muscle cells, Genes Dev. 18(22) (2004) 2730–2735. 10.1101/gad.308904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu H, Kennard S, Lilly B, NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1, Circ Res 104(4) (2009) 466–475. 10.1161/CIRCRESAHA.108.184846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Economides AN, Frendewey D, Yang P, Dominguez MG, Dore AT, Lobov IB, Persaud T, Rojas J, McClain J, Lengyel P, Droguett G, Chernomorsky R, Stevens S, Auerbach W, DeChiara TM, Pouyemirou W, Cruz JM Jr., Feeley K, Mellis IA, Yasenchack J, Hatsell SJ, Xie L, Latres E, Huang L, Zhang Y, Pefanis E, Skokos D, Deckelbaum RA, Croll SD, Davis S, Valenzuela DM, Gale NW, Murphy AJ, Yancopoulos GD, Conditionals by inversion provide a universal method for the generation of conditional alleles, Proc. Natl. Acad. Sci. U. S. A 110(34) (2013) E3179–E3188. 1217812110 [pii]; 10.1073/pnas.1217812110 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zanotti S, Yu J, Sanjay A, Schilling L, Schoenherr C, Economides AN, Canalis E, Sustained Notch2 signaling in osteoblasts, but not in osteoclasts, is linked to osteopenia in a mouse model of Hajdu-Cheney syndrome, J. Biol. Chem 292(29) (2017) 12232–12244. 10.1074/jbc.M117.786129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ, DMP1-targeted Cre expression in odontoblasts and osteocytes, J. Dent. Res 86(4) (2007) 320–325. [DOI] [PubMed] [Google Scholar]
- [29].Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M, Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure, Proc. Natl. Acad. Sci. U. S. A 99(10) (2002) 7142–7147. 10.1073/pnas.102650499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML, VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells, Dev. Dyn 235(3) (2006) 759–767. 10.1002/dvdy.20643 [DOI] [PubMed] [Google Scholar]
- [31].Canalis E, Zanotti S, Beamer WG, Economides AN, Smerdel-Ramoya A, Connective Tissue Growth Factor Is Required for Skeletal Development and Postnatal Skeletal Homeostasis in Male Mice, Endocrinology 151(8) (2010) 3490–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, Carte J, Chen W, Roark N, Ranganathan S, Ravinder N, Chesnut JD, Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection, J. Biotechnol 208 (2015) 44–53. 10.1016/j.jbiotec.2015.04.024 [DOI] [PubMed] [Google Scholar]
- [33].Burger A, Lindsay H, Felker A, Hess C, Anders C, Chiavacci E, Zaugg J, Weber LM, Catena R, Jinek M, Robinson MD, Mosimann C, Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes, Development 143(11) (2016) 2025–2037. 10.1242/dev.134809 [DOI] [PubMed] [Google Scholar]
- [34].Paix A, Folkmann A, Rasoloson D, Seydoux G, High Efficiency, Homology-Directed Genome Editing in Caenorhabditis elegans Using CRISPR-Cas9 Ribonucleoprotein Complexes, Genetics 201(1) (2015) 47–54. 10.1534/genetics.115.179382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tang SH, Silva FJ, Tsark WM, Mann JR, A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ, Genesis 32(3) (2002) 199–202. 10.1002/gene.10030 [pii] [DOI] [PubMed] [Google Scholar]
- [36].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R, Guidelines for assessment of bone microstructure in rodents using micro-computed tomography, J. Bone Miner. Res 25(7) (2010) 1468–1486. [DOI] [PubMed] [Google Scholar]
- [37].Glatt V, Canalis E, Stadmeyer L, Bouxsein ML, Age-Related Changes in Trabecular Architecture Differ in Female and Male C57BL/6J Mice, J. Bone Miner. Res 22(8) (2007) 1197–1207. [DOI] [PubMed] [Google Scholar]
- [38].Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM, Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee, J. Bone Miner. Res 28(1) (2013) 2–17. 10.1002/jbmr.1805 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Halleux C, Kramer I, Allard C, Kneissel M, Isolation of mouse osteocytes using cell fractionation for gene expression analysis, Methods Mol. Biol 816 (2012) 55–66. 10.1007/978-1-61779-415-5_5 [doi] [DOI] [PubMed] [Google Scholar]
- [40].Canalis E, Schilling L, Zanotti S, Effects of Sex and Notch Signaling on the Osteocyte Cell Pool, J. Cell. Physiol 232(2) (2017) 363–370. 10.1002/jcp.25433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nazarenko I, Pires R, Lowe B, Obaidy M, Rashtchian A, Effect of primary and secondary structure of oligodeoxyribonucleotides on the fluorescent properties of conjugated dyes, Nucleic Acids Res. 30(9) (2002) 2089–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nazarenko I, Lowe B, Darfler M, Ikonomi P, Schuster D, Rashtchian A, Multiplex quantitative PCR using self-quenched primers labeled with a single fluorophore, Nucleic Acids Res. 30(9) (2002) e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lardelli M, Williams R, Mitsiadis T, Lendahl U, Expression of the Notch 3 intracellular domain in mouse central nervous system progenitor cells is lethal and leads to disturbed neural tube development, Mech Dev 59(2) (1996) 177–190. 10.1016/0925-4773(96)00589-8 [DOI] [PubMed] [Google Scholar]
- [44].Kouadjo KE, Nishida Y, Cadrin-Girard JF, Yoshioka M, St-Amand J, Housekeeping and tissue-specific genes in mouse tissues, BMC Genomics 8 (2007) 127. 10.1186/1471-2164-8-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Boucher J, Gridley T, Liaw L, Molecular pathways of notch signaling in vascular smooth muscle cells, Front Physiol 3 (2012) 81. 10.3389/fphys.2012.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW, Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene, Bone 35(1) (2004) 74–82. 10.1016/j.bone.2004.03.006 [doi];S8756328204001097 [pii] [DOI] [PubMed] [Google Scholar]
- [47].Couasnay G, Madel MB, Lim J, Lee B, Elefteriou F, Sites of Cre-recombinase activity in mouse lines targeting skeletal cells, J. Bone Miner. Res 36(9) (2021) 1661–1679. 10.1002/jbmr.4415 [DOI] [PubMed] [Google Scholar]
- [48].Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA, Matrix-embedded cells control osteoclast formation, Nat. Med 17(10) (2011) 1235–1241. nm.2448 [pii]; 10.1038/nm.2448 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H, Evidence for osteocyte regulation of bone homeostasis through RANKL expression, Nat. Med 17(10) (2011) 1231–1234. nm.2452 [pii]; 10.1038/nm.2452 [doi] [DOI] [PubMed] [Google Scholar]
- [50].Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y, The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development, J. Dent. Res 82(10) (2003) 776–780. [DOI] [PubMed] [Google Scholar]
- [51].Xu C, Dinh VV, Kruse K, Jeong HW, Watson EC, Adams S, Berkenfeld F, Stehling M, Rasouli SJ, Fan R, Chen R, Bedzhov I, Chen Q, Kato K, Pitulescu ME, Adams RH, Induction of osteogenesis by bone-targeted Notch activation, eLife 11 (2022) 10.7554/eLife.60183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Soares R, Balogh G, Guo S, Gartner F, Russo J, Schmitt F, Evidence for the notch signaling pathway on the role of estrogen in angiogenesis, Mol. Endocrinol 18(9) (2004) 2333–2343. 10.1210/me.2003-0362 [DOI] [PubMed] [Google Scholar]
- [53].Fan JZ, Yang L, Meng GL, Lin YS, Wei BY, Fan J, Hu HM, Liu YW, Chen S, Zhang JK, He QZ, Luo ZJ, Liu J, Estrogen improves the proliferation and differentiation of hBMSCs derived from postmenopausal osteoporosis through notch signaling pathway, Mol. Cell. Biochem 392(1–2) (2014) 85–93. 10.1007/s11010-014-2021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Farr JN, Roforth MM, Fujita K, Nicks KM, Cunningham JM, Atkinson EJ, Therneau TM, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S, Effects of Age and Estrogen on Skeletal Gene Expression in Humans as Assessed by RNA Sequencing, PLoS One 10(9) (2015) e0138347. 10.1371/journal.pone.0138347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Canalis E, Clinical and experimental aspects of notch receptor signaling: Hajdu-Cheney syndrome and related disorders, Metabolism 80 (2018) 48–56. 10.1016/j.metabol.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Adami G, Rossini M, Gatti D, Orsolini G, Idolazzi L, Viapiana O, Scarpa A, Canalis E, Hajdu Cheney Syndrome; report of a novel NOTCH2 mutation and treatment with denosumab, Bone 92 (2016) 150–156. 10.1016/j.bone.2016.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sivaraj KK, Adams RH, Blood vessel formation and function in bone, Development 143(15) (2016) 2706–2715. 10.1242/dev.136861 [DOI] [PubMed] [Google Scholar]
- [58].Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA, Niedzwiedzki T, The role of vasculature in bone development, regeneration and proper systemic functioning, Angiogenesis 20(3) (2017) 291–302. 10.1007/s10456-017-9541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kusumbe AP, Ramasamy SK, Adams RH, Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone, Nature 507(7492) (2014) 323–328. 10.1038/nature13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B, Notch3 is critical for proper angiogenesis and mural cell investment, Circ Res 107(7) (2010) 860–870. 10.1161/CIRCRESAHA.110.218271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mae MA, Jin S, Betsholtz C, Lendahl U, Notch3 is necessary for blood vessel integrity in the central nervous system, Arterioscler Thromb Vasc Biol 35(2) (2015) 409–420. 10.1161/ATVBAHA.114.304849 [DOI] [PubMed] [Google Scholar]
- [62].Baeten JT, Lilly B, Notch Signaling in Vascular Smooth Muscle Cells, Adv. Pharmacol 78 (2017) 351–382. 10.1016/bs.apha.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li L, Miano JM, Cserjesi P, Olson EN, SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis, Circ Res 78(2) (1996) 188–195. 10.1161/01.res.78.2.188 [DOI] [PubMed] [Google Scholar]


