Figure 1. Patient case example, clinical timeline, and tumor characteristics of Lynch syndrome patients with subsequent primary malignancies (SPM) after immune checkpoint blockade (ICB).
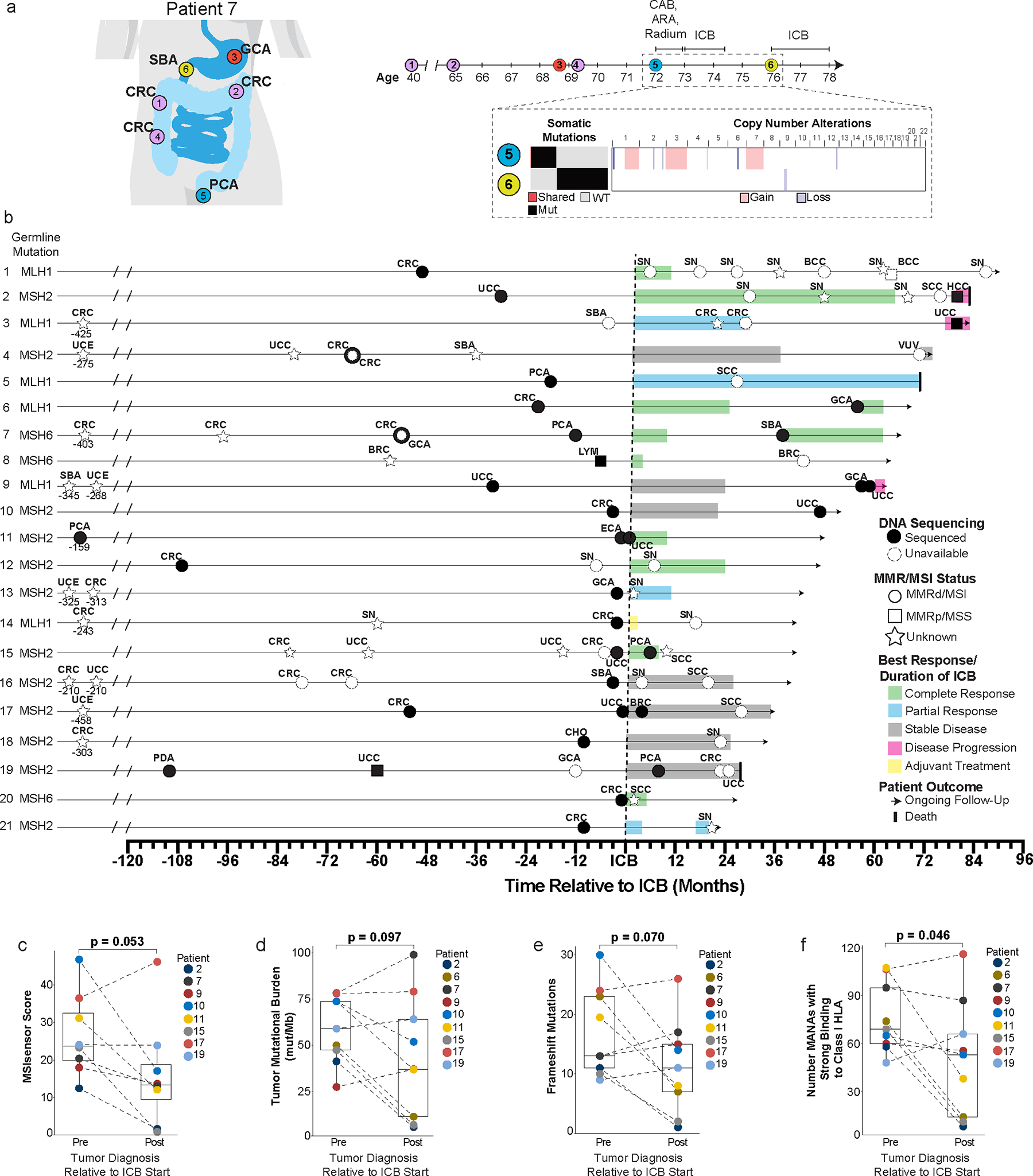
a, Case example of patient with multiple primary malignancies pre-ICB exposure. (Patient 7 from Figure 1b; Extended Data Table 1) Timeline of cancer diagnoses and sequential local and systemic interventions including combined androgen blockade (CAB), androgen receptor antagonist (ARA) and ICB. Targeted next-generation sequencing (MSK-IMPACT) comparing pre-ICB prostate cancer (PCA) and post-ICB small bowel cancer (SBA) by tumor mutational burden, somatic mutational profile, and copy-number alterations. Single shared somatic mutation, PTPRT at chromosome 20.
b, Timeline for SPM development post-ICB exposure (n=21 patients) inclusive of: SBA: Small bowel, CRC: Colorectal, GCA: Gastric, PCA: Prostate, SN: Sebaceous neoplasm, HCC: Hepatocellular, UCC: Urothelial, SCC: Squamous cell, CHO: Cholangiocarcinoma, ECA: Esophageal, BRC: Breast, LYM: Lymphoma, UCE: Uterine, PDA: Pancreatic, VUV: Vulvar.
c-f, MSISensor score (c), tumor mutational burden (d), frameshift mutations (e), and comparative analysis of strong binders to HLA Class 1 MHC (f) between pre- and post-ICB tumors. P-values are derived from pairwise t-testing for the paired pre and post tumor from each patient without replicates for each separate analysis.
Analyses included nine patients with 9 pre and 9 post tumors except analysis c (MSISensor score) which included 8 patients with 8 pre and 8 post tumors due to an MSI score not available for patient 6.
*MSI status on 1 sample with inadequate tumor purity was confirmed via MiMSI
