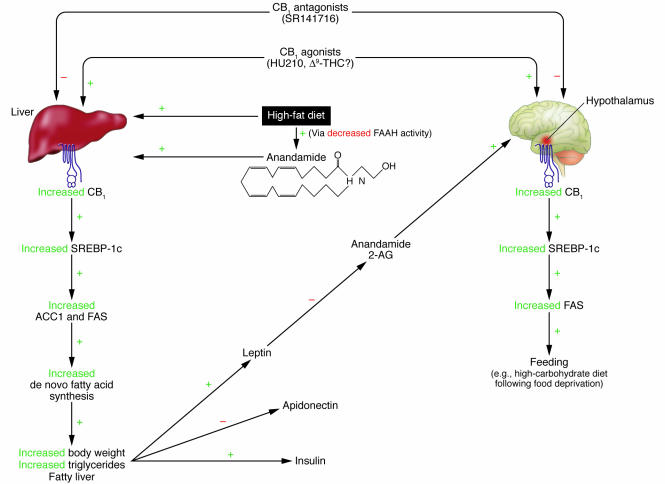
Figure 1.
Proposed model for the dual role of the endocannabinoid-mediated pathway in regulation of peripheral metabolic and central appetitive processes in liver and hypothalamus. In liver (left), a high-fat diet leads to increased CB1 levels as well as increased levels of anandamide, the latter of which result from decreased activity of FAAH, the primary enzyme responsible for this endocannabinoid’s catabolism. CB1 stimulation increases expression of the transcription factor SREBP-1c and its associated enzymes, ACC1 and FAS. Stimulation of this pathway leads to a functional increase in the rate of de novo fatty acid synthesis in liver and the increased occurrence of fatty liver and obesity. Through either direct or indirect mechanisms, this pathway also regulates plasma levels of hormones associated with metabolism and feeding, including insulin, leptin, and adiponectin. These altered levels of hormones may negatively affect feeding behavior and metabolism. For example, exogenous administration of leptin leads to decreased levels of anandamide and 2-AG in the hypothalamus and consequently inhibits feeding behavior. Conversely, stimulation of CB1 in the hypothalamus (right) activates SREBP-1c and FAS, which leads to a hyperphagic response to a high-carbohydrate meal following a fast. Disruption of CB1 signaling through the use of CB1_/_ mice or SR141716 (Rimonabant) administration blocks both pathways, leading to a net effect of decreased fatty acid production and prevention of hyperphagia.