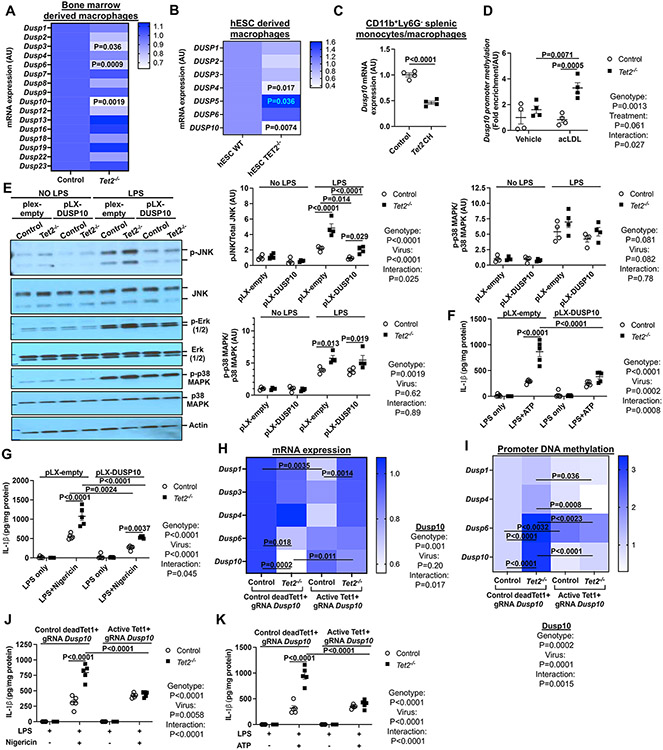
Figure. 2. Dusp10 downregulation due to elevated promoter methylation drives JNK and inflammasome activation in TET2−/− macrophages.
(A) qPCR analysis of dual phosphatases in control and Tet2−/− BMDMs. (B) qPCR analysis of DUSP genes in WT and TET2−/− human embryonic stem cell (hESC)-derived macrophages. (C) Dusp10 mRNA expression in Ly6G−CD11b+ monocytes/macrophages isolated from Ldlr−/− mice that were transplanted with bone marrow mixture of WT or 10%Tet2−/−/90%WT and fed with chow or WTD for 4 weeks. (D) meDIP analysis of Dusp10 promoter methylation in vehicle and acLDL loaded control and Tet2−/− BMDMs. (E-G) Control and Tet2−/− BMDMs were infected with control (pLX-empty) or DUSP10 lentiviruses for 72h. (E) Immunoblot analysis of JNK (Thr183/Tyr185), Erk (1/2) and p38 MAPK phosphorylation from cells primed with LPS for 15 min (F) IL-1β secretion from LPS+ATP. (G) IL-1β secretion from LPS+Nigericin. (H-K) Control and Tet2−/− BMDMs were infected with lentiviruses expressing deadCas9-Tet1 (active dC-T) or deadCas9-a catalytically dead form of Tet1 (control dC-dT) with gRNAs targeting the Dusp10 promoter region for 72 hours. (H) qPCR analysis of Dusp genes. (I) meDIP analysis of Dusp promoter methylation. (J) IL-1β secretion from LPS+Nigericin. (K) IL-1β secretion from LPS+ATP. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by t-test (A-C) and two-way ANOVA with Sidak’s multiple comparison test (D-K).