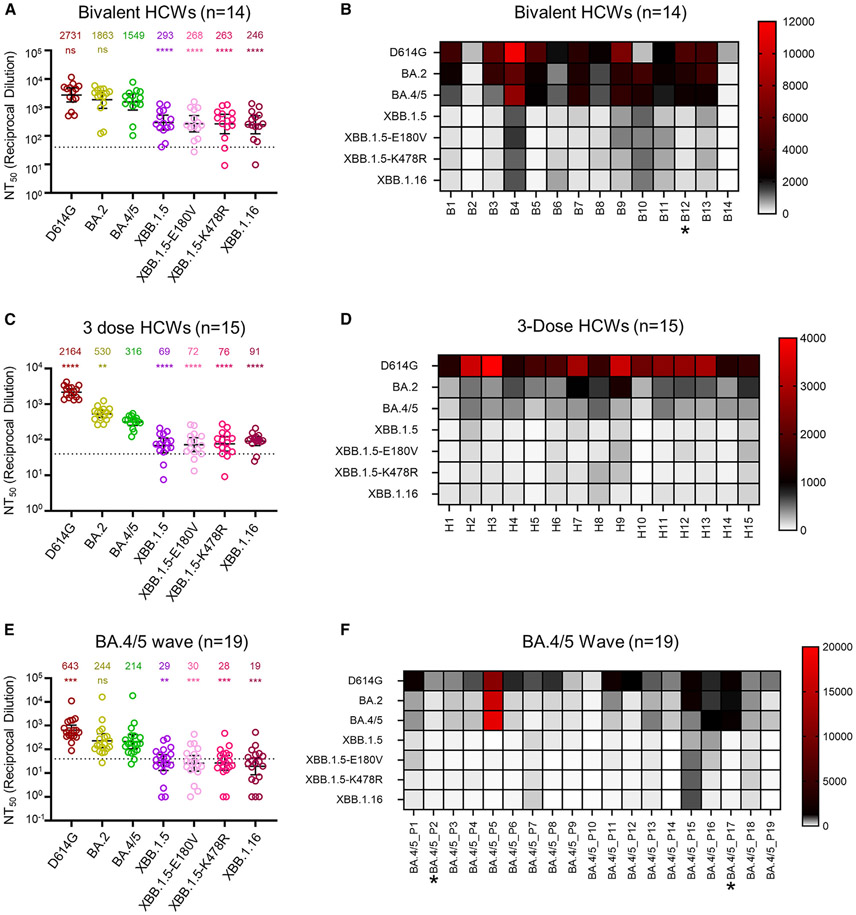
Figure 2. Neutralization of XBB.1.16 by bivalent-vaccinated, 3-dose-vaccinated, and BA.4/5 convalescent sera.
Neutralizing antibody titers of three different cohorts of human sera against lentiviral pseudotypes bearing XBB.1.16 or other S proteins of interest were determined.
HCWs receiving 3 doses of monovalent mRNA vaccine plus 1 dose of bivalent mRNA vaccine (n = 14) (A), HCWs receiving 3 doses of monovalent mRNA vaccine (n = 15) (C and D), and first responders and household contacts that tested positive for COVID-19 during the BA.4/5 wave of infection (E and F) are all from Columbus, OH (USA). Bars represent geometric means with 95% confidence intervals. Geometric mean NT50 values are displayed for each subvariant on the top (A, C, and E). Heatmap depictions of neutralizing antibody titers were made for individuals in the (B) bivalent-vaccinated, (D) 3-dose-vaccinated, and (F) BA.4/5-wave-infected cohorts. Statistical significance in (A), (C), and (E) was determined using log10-transformed NT50 values to better approximate normality. Comparisons between multiple groups were made using a one-way ANOVA with Bonferroni post-test, with all compared to BA.4/5 in each panel. Dashed lines represent the assay’s limit of detection, i.e., 40 (the lowest dilution factor of serum samples). Asterisk in (B) indicates the individual infected by SARS-CoV-2 within 6 months before the sera sample collection, and asterisks in (F) indicate the individuals who had received three doses of mRNA vaccine before infection. p values are displayed as “ns” p > 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.