Fig. 2. Repeated CNO administration results in diminished baseline and AMPH-induced locomotion in TH-CrehM3D(Gq) mice that is reversed after CNO washout.
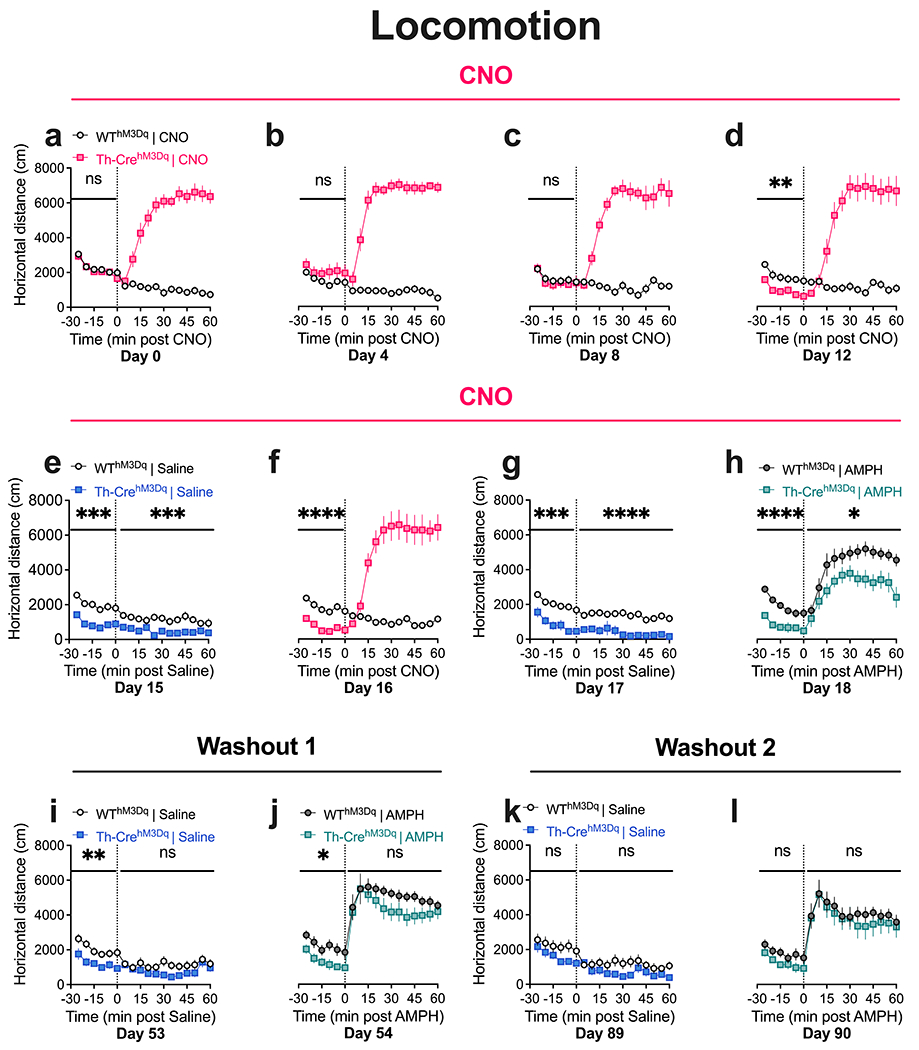
(a) TH-CrehM3Dq mice display increased open-field locomotor activity following acute CNO administration. No between-genotype difference is observed in baseline locomotion on Day 0. (b-d) TH-CrehM3Dq mice show stable increases in locomotion following repeated CNO injections. A decrease in baseline locomotion is observed in TH-CrehM3Dq mice on Day 12. (e-h) The decreases in baseline locomotion in TH-CrehM3Dq mice is again observed on Days 15-18 of the CNO phase. Decreases in locomotor activity is also observed after saline administration (e, g). TH-CrehM3Dq mice show diminished AMPH-induced hyperlocomotion after repeated CNO (h). (i, j) TH-CrehM3Dq mice show diminished baseline locomotion after one month of CNO washout. No between-genotype differences are observed in AMPH-induced hyperlocomotion after one month washout. (k, l) TH-CrehM3Dq mice show comparable baseline and AMPH-induced hyperlocomotor response to WThM3Dq mice after two months of CNO washout. N = 8 TH-CrehM3Dq and 10 WThM3Dq mice. Main effect of genotype, ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05; nsP, not significant. Also see Supplemental Information Fig. SI1 for Area Under Curve (AUC) analysis of this dataset.
