Figure 3. YTHDF1 condenses with the ribosome to promote mRNA translation.
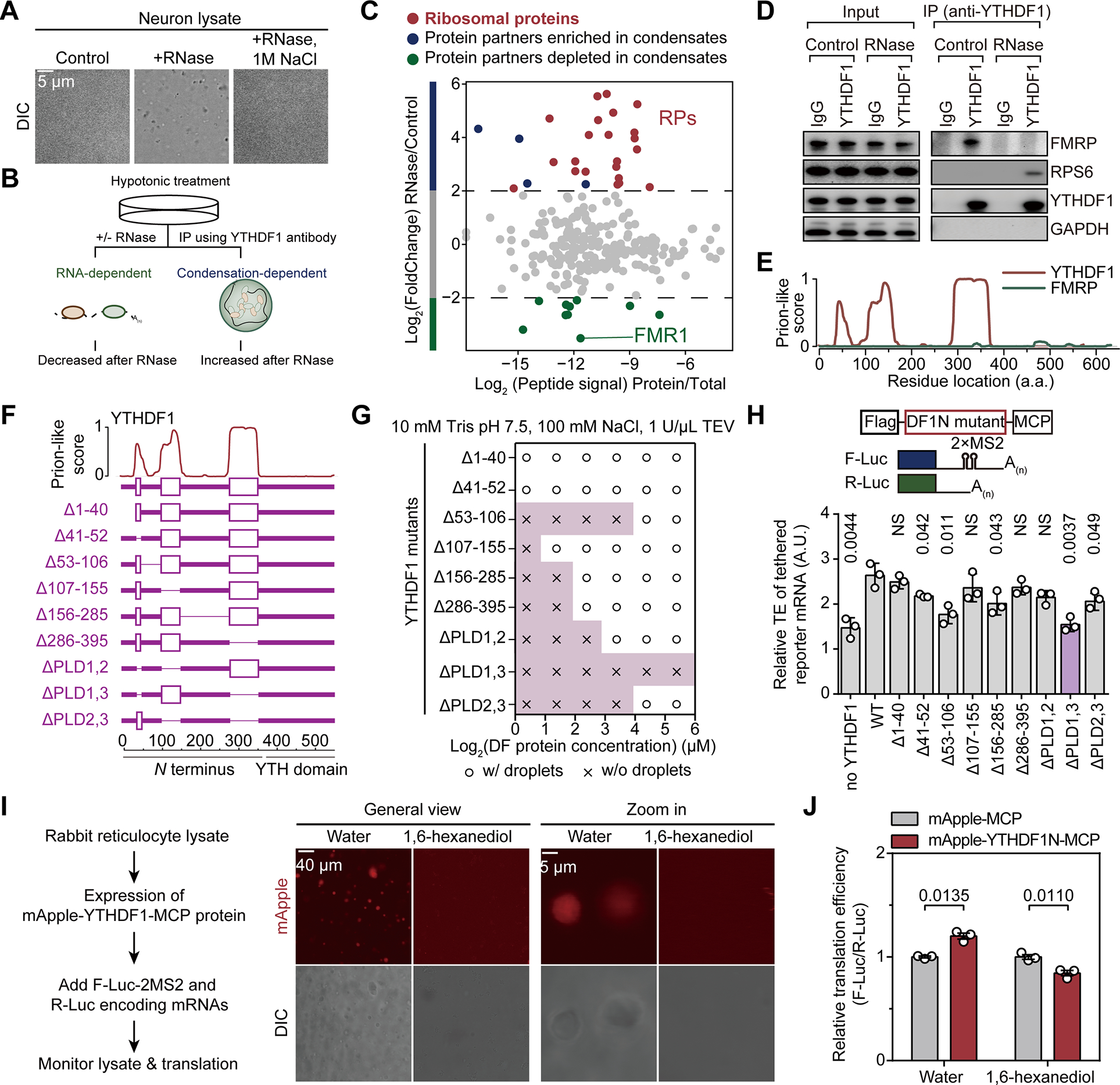
(A) Representative images of mouse neuron lysates in controls samples, samples treated by RNase, or samples treated by RNase and NaCl (n = 3).
(B) Schematic of using RNase to precipitate prion-like proteins.
(C) Protein partners of YTHDF1 revealed by co-immunoprecipitation with RNase treatment.
(D) Western blot analysis of YTHDF1 protein partners with RNase treatment.
(E) Illustration of the prion-like features of YTHDF1 and FMRP calculated by PLAAC.
(F) Illustration of YTHDF1 mutants with deletion of different domains.
(G) Granule formation propensity of YTHDF1 mutants.
(H) Relative translation efficiency measured by reporter assay showing effects of YTHDF1 mutants on mRNA translation (n = 3 for each condition).
(I) In vitro translation assay with rabbit reticulocyte lysate (RRL) and YTHDF1 (n = 3 each condition). Representative fluorescence images were shown to demonstrate formations of YTHDF1 granules and that 0.2% (w/v) 1,6-hexanediol dissolved them.
(J) Bar plots showing the translation efficiency of YTHDF1-tethered reporter mRNA normalized to untethered control.
Data are mean ± s.e.m. P values were determined using a two-tailed t test with Welch’s correction. NS means not significant (P values > 0.05). Reported P values are for the individual comparisons.
