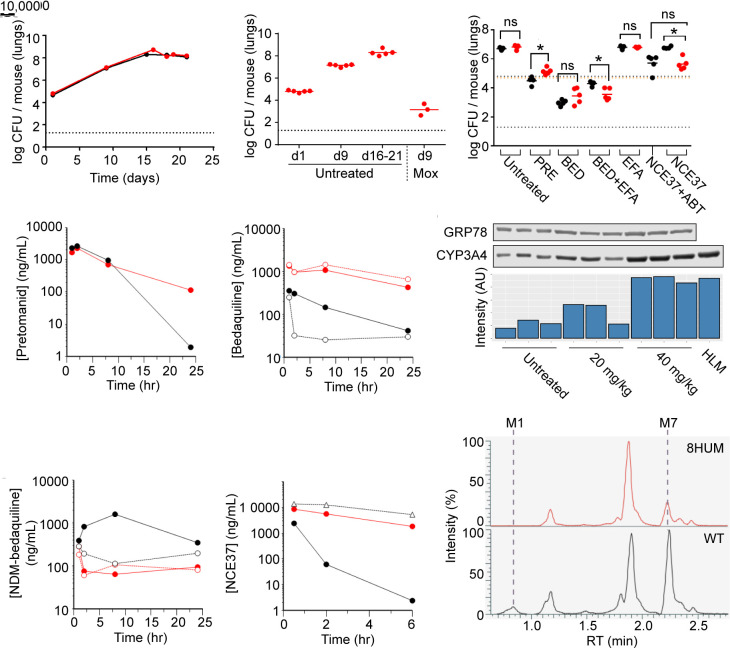
Fig. 5.
Clinically relevant DDI and active metabolite levels are captured within compound efficacy assessments in an 8HUM model of tuberculosis infection. (A) 8HUM (red) and WT (black) mice were intratracheally infected with M. tuberculosis and lungs collected at the indicated timepoints for assessment of bacterial burden in lung tissue by CFU assay. (B) The effect of moxifloxacin treatment (30 mg/kg QD) on lung CFU assay results in 8HUM infected with M. tuberculosis. (C) The effect of pretomanid, bedaquiline, and NCE37 treatment on lung CFU assay results in 8HUM (red) and WT (black) mice infected with M. tuberculosis. Blood levels of (D) pretomanid and (E) bedaquiline in 8HUM and WT were assessed on the final day of dosing (8HUM: filled red circles, WT: filled black circles, 8HUM+efavirenz: open red circles, WT+efavirenz: open black circles, WT+ABT: open black triangles). (F) Western blot and chemiluminescence signal intensity showing the effect of 20 and 40 mg/kg QD efavirenz on the hepatic level of CYP3A4 in 8HUM. Blood levels of (G) N-desmethyl bedaquiline and (H) NCE37 in 8HUM and WT were assessed on the final day of dosing (symbols as for (D) and (E)). (I) All-component XIC for putative metabolites of NCE37 in blood samples from 8HUM (red) and WT (black) taken 1 h after compound administration.