Figure 1. Flow of Participants in the KARDIA-1 Trial of Subcutaneous Zilebesiran for Hypertension.
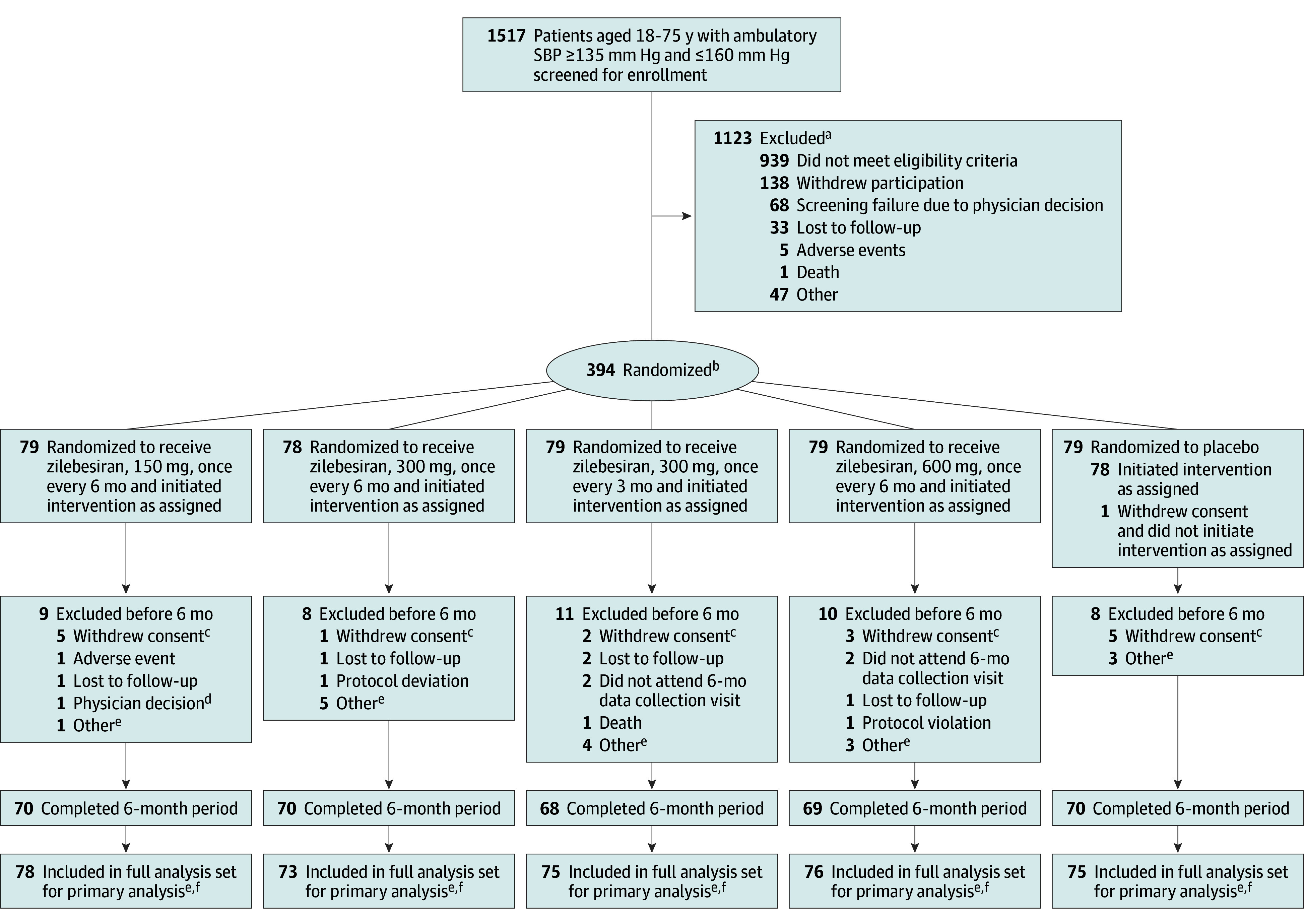
aA patient could have multiple reasons for screen failure and was counted for each reason separately.
bRandomization was stratified by race (Black or other) and baseline mean 24-hour systolic blood pressure (<145 or ≥145 mm Hg).
cPatients withdrew consent because of work-related reasons (n = 4), distance to the site (n = 3), no disclosed reason (n = 2), principal care clinician’s advice (n = 2), time constraints (n = 1), the study not being worth their time (n = 1), personal reasons (n = 1), unwillingness to adhere to ambulatory blood pressure monitoring requirements (n = 1), and adverse events (n = 1).
dPatient was incarcerated during study.
ePatients enrolled at sites in Ukraine (n = 16) were excluded after randomization because war prevented continued data collection.
fPrimary analysis was carried out in the full analysis set, which included all randomized patients who received any amount of study drug.
