Figure 1.
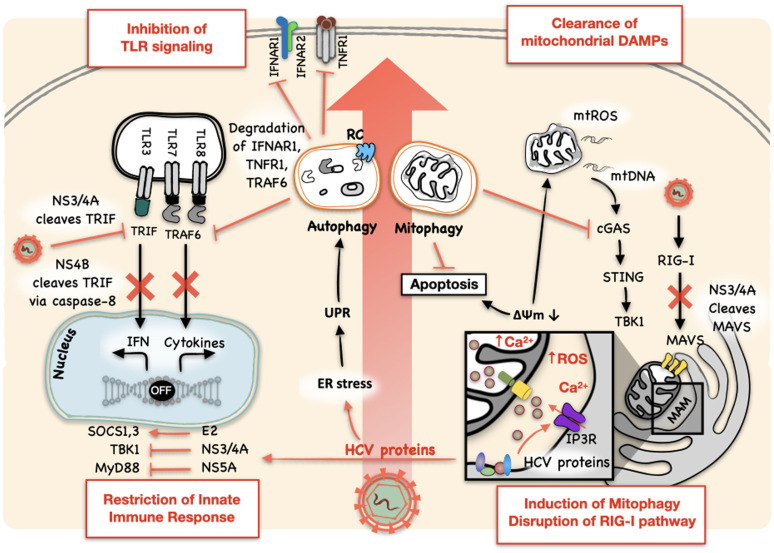
HCV inhibits the innate immune response. HCV proteins can directly inhibit the innate immune response. NS3/4A plays multiple roles in blocking the host innate immune response. It cleaves MAVS on MAM to block the RIG-I signaling pathway and TRIF to inhibit the TLR3 signaling pathway. NS4B can also disrupt TLR3-mediated IFN signaling by inducing the degradation of TRIF via caspase-8. Other HCV proteins had also been shown to regulate and restrict the production of IFNs and their antiviral signaling: E2 induces SOCS1 and SOCS3; NS3/4A sequesters STING to inhibit the activation of TBK1; NS5A sequesters MyD88 to inhibit TLR signaling; and NS5B induces and activates PKR to inhibit the expression of IFNs and ISGs. HCV also usurps the antiviral activity of autophagy and uses it for its own replication. It not only utilizes the autophagic membranes as the sites for its RNA replication and the production of infectious HCV particles, but also manipulates autophagy to regulate type I IFN response by promoting the autophagic degradation of IFNAR1, TNFR1, and TRAF6, important signal transducers for the activation of NF-κB and the expression of pro-inflammatory cytokines. HCV-induced mitophagy also regulates innate immune response by clearing mitochondrial DAMPs, namely mtROS and mtDNA, and damaged mitochondria themselves, which otherwise would activate the cGAS/STING/TBK1 pathway to induce the expression of IFNs. Mitophagy also inhibits premature apoptotic cell death and promotes HCV persistence. RC: HCV RNA replication complex. The mitochondria-associated membrane (MAM) is boxed and enlarged in the inset to reveal the Ca2+ transporter IP3R.