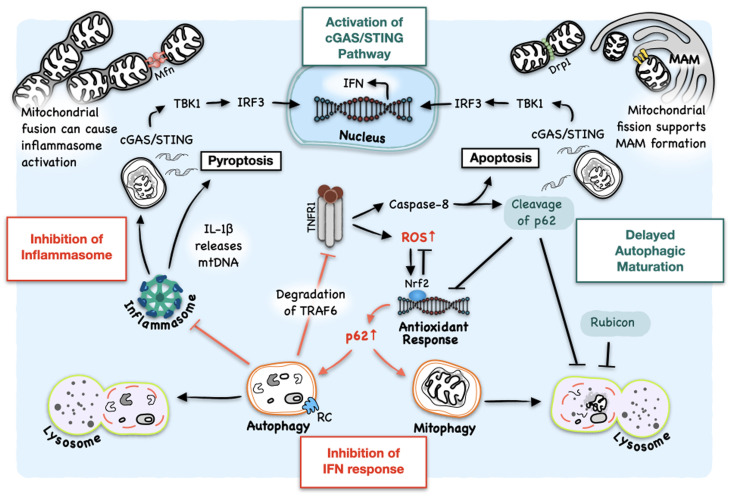
Figure 2.
Interplay between HCV-induced autophagy and host innate immune response. HCV-induced autophagy removes DAMPs to inhibit the formation of inflammasomes and hence pyroptosis. HCV-induced autophagic degradation of TNFR1 also inhibits the activation of caspase-8 and apoptosis. However, host cells in response to HCV infection also express TNF-α and IFNAR2 (also see Figure 1 ). TNF-α at a low concentration causes the generation of ROS to initiate autophagy. However, it, at a high concentration, can also put a halt on the completion of autophagy via caspase-8-mediated cleavage of p62, ultimately activating the cGAS/STING/TBK1 pathway and apoptosis. TNF-α-induced cleavage of p62 also disrupts Nrf2 signaling, which is activated by HCV to induce the expression of antioxidant genes to alleviate oxidative stress. HCV also delays the maturation of autophagosomes by inducing the expression of Rubicon for the accumulation of autophagosomes to support its RNA replication, which can also lead to the accumulation of DAMPs. Mitochondrial dynamics are closely related to the regulation of innate immune response. On the other hand, mitochondrial fusion supports inflammasome activation and pro-survival pathways that, if prolonged, can contribute to tumorigenesis. On the other hand, mitochondrial fission, which is coupled with mitophagy and promoted by HCV, supports the formation of MAM which can serve as the platform to mediate IFN response. RC: HCV replication complex.