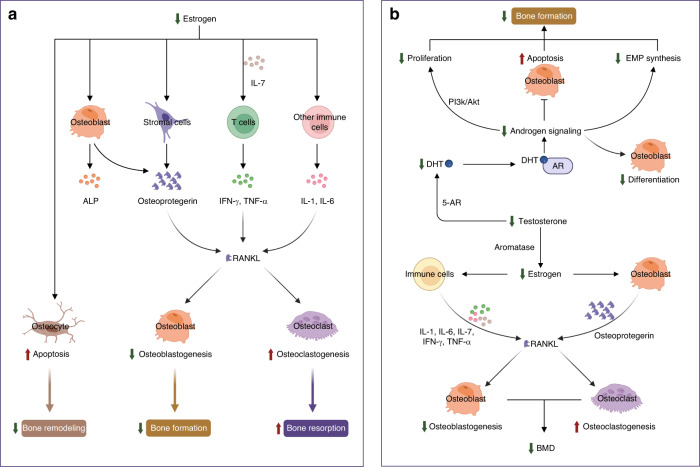
Fig. 3.
Mechanisms of sex steroid hormones on bone homeostasis. a Mechanism of estrogen action on bone cells. Estrogen is the most important sex hormone in women who undergo a hormonal shift in 17β-estradiol levels, transitioning from perimenopause to early postmenopause. The declining estrogen levels after menopause directly enhance the apoptosis of osteocytes to reduce bone remodeling, indirectly decrease osteoblastogenesis, and increase osteoclastogenesis by regulating RANKL. Estrogen regulates RANKL by acting on stromal cells and immune cells by changing cytokine profiles. b Schematic of the effects of male sex hormones on bone cells. The male sex hormone testosterone is an important regulator of bone cells, mainly osteoblasts and osteoclasts. Decreased testosterone levels in older men induce a decrease in DHT, which further represses the proliferation and differentiation of osteoblasts, increases apoptosis of osteoblasts, and reduces the synthesis of EMP. The decline in testosterone results in reduced estrogen levels, which further directly or indirectly decreases osteoblastogenesis and increases osteoclastogenesis to reduce BMD. ALP alkaline phosphatase, Bcl-2 B-cell lymphoma-2, ERα estrogen receptor alpha, ERβ estrogen receptor beta, FasL Fas ligand, IL-1 interleukin-1, IL-6 interleukin-6, IL-7 interleukin-7, IFN-γ interferon-γ, RANKL receptor activator of nuclear factor kappa B ligand, TNF-α tumor necrosis factor-alpha, Akt serine/threonine-protein kinase, AR androgen receptor, 5-AR congenital 5-alpha-reductase, BMD bone mineral density, DHT dihydrotestosterone, EMP erythromyeloid progenitor, PI3K phosphatidylinositol-3 kinase