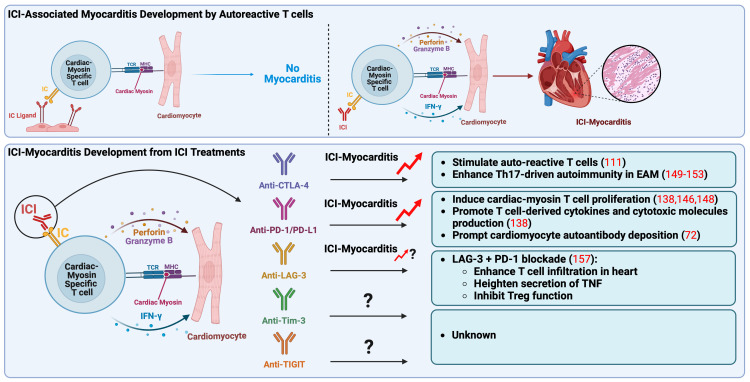
Figure 1.
Pathogenic mechanisms of immune checkpoint inhibitors associated myocarditis. Autoreactive T cells, which recognize cardiac myosin heavy chain, main autoantigen in the heart, play a crucial role in the onset of ICI-myocarditis. These autoreactive T cells may be present in the heart in a naïve state due to impaired thymic selection, expressing elevated levels of IC as a peripheral tolerance mechanism to prevent their activation. In a mouse model, ICI treatment seems to directly activate these autoreactive T cells by obstructing the inhibitory IC pathway, specifically targeting the heart. Recent reports have indicated that anti-CTLA-4 and anti-PD-1/PD-L1 treatments result in the clonal expansion of T cells specific to cardiac myosin, observed in peripheral blood mononuclear cells of patients with ICI-associated myocarditis, indicating their pathogenic role in clinical scenarios. Anti-CTLA-4 is associated with the promotion of Th17-mediated autoimmunity in EAM, while anti-PD-1/PD-L1 is linked to excessive production of T cell derived cytokines and cytotoxic molecules like IFN-γ, perforin, and granzyme B, ultimately contributing to myocarditis development. Notably, anti-LAG-3 has only been associated with ICI-myocarditis when combined with anti-PD-1. CTLA-4, cytotoxic T-lymphocyte antigen-4; EAM, experimental autoimmune myocarditis; IC, immune checkpoint; ICI, immune checkpoint inhibitor; IFN-γ, interferon-γ; LAG-3, lymphocyte activation gene-3; MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, programmed death ligand-1, TCR, T-cell receptor; Th17, IL-17-producing T cell (type 17 helper T cell); TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domain; Tim-3, T cell immunoglobulin and mucin domain-containing protein 3; TNF, tumor necrosis factor; Treg, regulatory T lymphocytes.