Abstract
Diploscapter, a thermotolerant, free-living soil bacterial-feeding nematode commonly found in compost, sewage, and agricultural soil in the United States, was studied to determine its potential role as a vehicle of Salmonella enterica serotype Poona, enterohemorrhagic Escherichia coli O157:H7, and Listeria monocytogenes in contaminating preharvest fruits and vegetables. The ability of Diploscapter sp. strain LKC25 to survive on agar media, in cow manure, and in composted turkey manure and to be attracted to, ingest, and disperse food-borne pathogens inoculated into soil or a mixture of soil and composted turkey manure was investigated. Diploscapter sp. strain LKC25 survived and reproduced in lawns of S. enterica serotype Poona, E. coli O157:H7, and L. monocytogenes on agar media and in cow manure and composted turkey manure. Attraction of Diploscapter sp. strain LKC25 to colonies of pathogenic bacteria on tryptic soy agar within 10, 20, 30, and 60 min and 24 h was determined. At least 85% of the worms initially placed 0.5 to 1 cm away from bacterial colonies migrated to the colonies within 1 h. Within 24 h, ≥90% of the worms were embedded in colonies. The potential of Diploscapter sp. strain LKC25 to shed pathogenic bacteria after exposure to bacteria inoculated into soil or a mixture of soil and composted turkey manure was investigated. Results indicate that Diploscapter sp. strain LKC25 can shed pathogenic bacteria after exposure to pathogens in these milieus. They also demonstrate its potential to serve as a vector of food-borne pathogenic bacteria in soil, with or without amendment with compost, to the surface of preharvest fruits and vegetables in contact with soil.
Associations between minimally processed fruits and vegetables and improved health, including the prevention of certain cancers, have stimulated an increase in per capita consumption of fresh produce (9). In the United States, outbreaks of human bacterial infections associated with consumption of produce have also increased in the past decade (13). These events have prompted scientists to identify factors that may contribute to contamination of preharvest and postharvest fruits and vegetables (5). Agronomic practices that may facilitate contamination of preharvest produce are of concern, and microbiota in soil microenvironments have been studied to determine their potential roles (10). A lack of appropriate hygienic practices in growing, harvesting, processing, distributing, and preparing fruits and vegetables eaten raw can have a substantial impact in contributing to increased risk of human microbial infections (6).
Numerous types of microorganisms inhabit the soil environment, but meso-fauna of particular interest with regard to their role as potential vectors of food-borne pathogens are free-living nematodes that feed on bacteria. Although many of these nematodes are universal in agricultural soils, very little is known about their potential role as disseminators of human pathogens that may be present as a result of application of manure, improperly treated irrigation water, or runoff water from nearby livestock operations. Free-living, bacterivorous nematodes are attracted to areas of high organic activity in soil, largely in the top 5 cm of soils, which contain decaying plant materials and to which animal manure is applied, where they ingest resident bacteria as a nutrient source (10).
Studies have shown that Caenorhabditis elegans is attracted to and ingests food-borne pathogens (1, 3, 10). A related free-living nematode found more commonly in the rhizosphere of agricultural soils is Diploscapter, several species of which are reported to be present in a range of agricultural habitats (14). In comparison to C. elegans, Diploscapter spp. have a markedly higher thermal tolerance (12). Agricultural practices involving the use of compost, manure, and poorly treated irrigation water result in increased numbers of nematodes (4, 11), but the behavior of Diploscapter nematodes in soil environments and their ability to feed on food-borne pathogens have not been described. The feeding behavior and reproductive cycles of Diploscapter nematodes and other free-living nematodes render them potential vectors for transporting and dispersing pathogenic bacteria in agricultural soil environments.
The primary objectives of this study were to determine survival and reproduction characteristics of Diploscapter nematodes fed on food-borne pathogenic bacteria; to determine if Diploscapter nematodes are attracted to pathogenic bacteria; and to determine if pathogenic bacteria ingested by Diploscapter nematodes or adhering to the worms in soil are subsequently shed and dispersed. Information gained in this study will be of value in assessing the potential role of Diploscapter nematodes as a vector of pathogenic bacteria to preharvest fruits and vegetables.
MATERIALS AND METHODS
Diploscapter sp. strain LKC25 culture.
Diploscapter sp. strain LKC25, morphologically similar to the root-associated species Diploscapter coronatus and Diploscapter pachys (4, 14), was obtained by one of the authors from greenhouse pot soil surrounding root-knot nematode-infested tomato roots in the Nematology Laboratory, United States Department of Agriculture Agricultural Research Service (USDA-ARS), Beltsville, MD. It was subcultured and maintained on nematode growth medium (NGM) agar on which a lawn of Escherichia coli strain OP50 had formed by incubating surface-inoculated plates for 24 h at (37°C) (8). Diploscapter sp. strain LKC25 used in experiments was harvested from plates incubated at 30°C for 7 days.
Nematode microscopy.
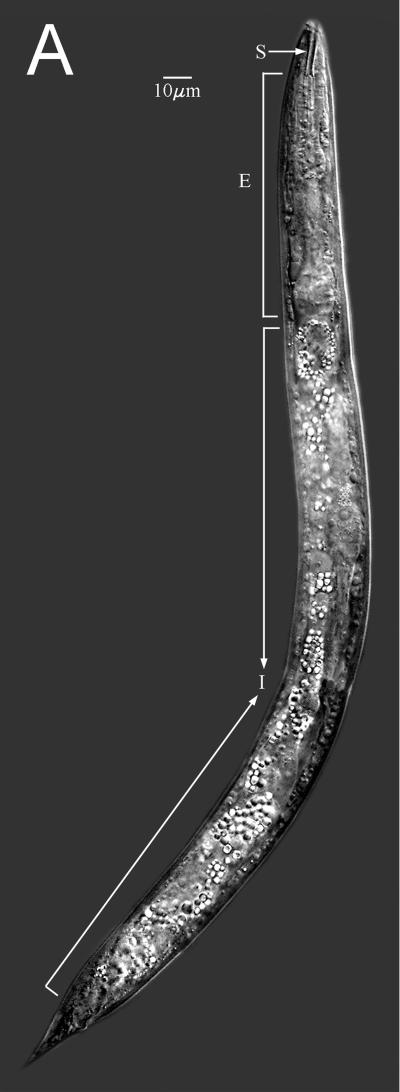
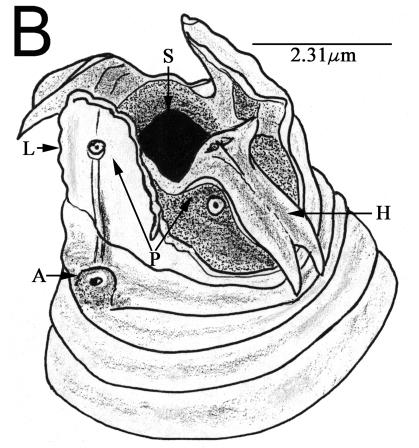
The lateral view light microscopic image (Fig. 1A) was made with a Zeiss Ultraphot microscope with differential interference contrast optics, Image Pro-Plus (Media Cybernetics, Silver Spring, Md.) screen capture, and stitched with Adobe PhotoShop (Adobe Systems Inc., San Jose, Calif.). Other nematodes were fixed in 3% glutaraldehyde in 0.05 M phosphate buffer, dehydrated in an ethanol series, critical point dried, individually mounted on aluminum stubs (15), and observed in a Hitachi S-4100 field emission scanning electron microscope. A 13,000× head image taken at 2 kV was redrawn for clarity of morphological structures (Fig. 1B).
FIG. 1.
Diploscapter sp. strain LKC25 full-body digital image (A) using differential interference contrast optics showing stoma (S), esophagus (E), and intestine (I), and a drawing from the scanning electron microscope image (B) of the anterior end showing stoma opening (S), subventral hamuli (H), lateral laciniae (L), sensory amphid (A), and labial papillae (P).
Bacterial strains.
Three food-borne pathogenic bacteria were used: Salmonella enterica serotype Poona (strain 01A4754, from a patient with salmonellosis associated with consuming cantaloupe), enterohemorrhagic Escherichia coli O157:H7 (strain SEA-13B88, from a patient infection associated with consuming apple cider), and Listeria monocytogenes (strain G1091, from coleslaw). Nonpathogenic E. coli OP50 was used as a control. The three pathogens and E. coli OP50 were grown in tryptic soy broth (TSB, pH 7.3) (Difco/BBL, Sparks, MD) supplemented with nalidixic acid (50 μg/ml) (TSBN) at 21°C, with transfers using loop inoculum (ca. 10 μl) at 24-h intervals. Cells from these cultures were used in all experiments. Stock cultures were stored at −20°C were thawed and cells grown in TSBN were held at 4°C between initiation of experiments. Storage at 4°C did not exceed 3 weeks.
Survival and growth of Diploscapter sp. strain LKC25 on agar media inoculated with bacteria.
Aliquots (0.1 ml) of 24-h cultures of S. enterica Poona, E. coli O157:H7, L. monocytogenes, and E. coli OP50 grown in TSBN were surface spread on NGM agar and tryptic soy agar (TSA, pH 7.3) (BBL/Difco) supplemented with nalidixic acid (50 μg/ml) (TSAN) and incubated at 21°C for 24 h, allowing bacterial lawns to develop. In the center of each 24-h bacterial lawn, 10 μl of K medium (contains, per liter of deionized water, 2.36 g of potassium chloride and 3.0 g of sodium chloride) (16) containing 40 to 50 Diploscapter sp. strain LKC25 worms was deposited. Worms were incubated at 30°C and examined for viability and increases in population after 3 and 10 days.
Survival of Diploscapter sp. strain LKC25 in cow manure and composted turkey manure.
Fresh cow manure was obtained from the College of Veterinary Medicine, University of Georgia, Athens, Ga. Samples (3 g) were placed in petri plates (35 mm diameter by 10 mm deep) along with 5 μl of K medium containing 20 to 25 Diploscapter sp. strain LKC25 worms and incubated at 30°C for 24 or 48 h. Worms were recovered from manure using a centrifugation-flotation technique in a colloidal silica suspension (Ludox extraction) and examined for viability using the American Society for Testing and Materials method (2) developed for C. elegans. Composted turkey manure was obtained from P. Millner at the USDA-ARS, Beltsville, MD. Samples were placed in small (35-mm diameter by 10-mm deep) petri dishes and inoculated with 20 to 25 Diploscapter sp. strain LKC25 worms. Worms were separated from the compost after incubating the mixture for 24 or 48 h at 30°C using the Ludox extraction method (2) and analyzed for viability.
In another series of experiments, composted turkey manure was amended into soil at a ratio of 1 g of compost to 2.33 g of soil, inoculated with 20 to 25 Diploscapter sp. strain LKC25, incubated for 24 and 48 h at 30°C, and analyzed for numbers of viable worms. Tifton soil (94% sand, 4% silt, 2% clay, 1.2% organic matter, and a pH of 5.6), obtained from the University of Georgia Soil Testing Laboratory, was used.
Attraction assays.
Two 10-μl aliquots of TSBN culture containing E. coli O157:H7, S. enterica serotype Poona, L. monocytogenes, or E. coli OP50 were deposited 1 cm apart on the surface of TSAN in petri dishes (60 mm diameter by 15 mm deep) and incubated at 21°C for 24 h. A suspension (5 μl) of Diploscapter sp. strain LKC25 (25 to 50 worms) in K medium was placed equidistant between the sites of inoculation. The location of the worms on the surface of TSAN incubated at 21°C for 10, 20, 30, and 60 min and 24 h was monitored using a computer-captured image technique (3).
Attraction assays using three test pathogens inoculated on the same TSAN plate were also conducted. Suspensions (10 μl) of 24-h TSBN cultures were deposited on the surface of TSAN at locations 2 cm apart. Diploscapter sp. strain LKC25 (25 to 50 worms in 5 μl of K medium) was deposited at a point on the plate equidistant from the three inoculated sites and monitored for location up to 24 h as described above.
Shedding of pathogenic bacteria.
Diploscapter sp. strain LKC25 (20 to 25 worms/2.33 g of soil) and pathogenic bacteria (5.8 to 6.2 log10 CFU/2.33 g of soil) were inoculated into soil amended with composted turkey manure (1 g of compost per 2.33 g of soil) as well as unamended soil, and incubated at 30°C for 24 or 48 h. Worms were separated from the soil and compost-amended soil as described above. Prior to transfer to agar plates, the worms were rapidly washed in K medium (10 ml in a 15-ml tube) three times. With each rinse, K medium was aspirated with air in the collection tube using a sterile pipette. The worms were allowed to settle to the bottom of the tube, and the supernatant was removed immediately. After the third rinse, the worms were resuspended in K medium, and a platinum wire was used to transfer two worms to the surface of TSAN plates for 2 h. The worms were removed, and the plates were incubated at 37°C for 24 h to allow bacterial colony formation. The number of CFU of each bacterium shed by the two worms was determined by counting the number of colonies formed on TSAN.
RESULTS AND DISCUSSION
Nematode morphology.
The lateral view of the nematode (Fig. 1A) shows a medial section with an anterior columnar stoma, muscular esophagus, and birefringent intestinal region. Under the scanning electron microscope, the anterior lips are elaborated into subventral bifurcate hamuli and lateral laciniae with scalloped lateral and apical margins (Fig. 1B) typical of this new species of Diploscapter.
Survival of Diploscapter sp. strain LKC25 as affected by medium and bacterium.
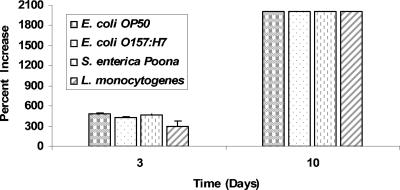
Diploscapter sp. strain LKC25 survived and reproduced on NGM agar inoculated with food-borne pathogenic bacteria or E. coli OP50 for at least 10 days (Fig. 2). The increase in initial number (approximately 40) of Diploscapter sp. strain LKC25 was largely unaffected by the type of test bacterium. Populations of the worm on NGM agar were too numerous to count, i.e., >900 worms/plate, at 10 days and remained at those populations for up to 30 days, regardless of the bacterium used as a nutrient source. The life cycle of this organism is believed to be similar to that of several related species and estimated to be 4 to 6 days (14). Within this 30-day period, several life cycles would have occurred.
FIG. 2.
Percent increase in number of Diploscapter worms deposited on NGM agar inoculated with E. coli OP50, E. coli O157:H7, S. enterica serotype Poona, or L. monocytogenes and incubated for 3 or 10 days at 30°C.
Survival in cow manure and composted turkey manure.
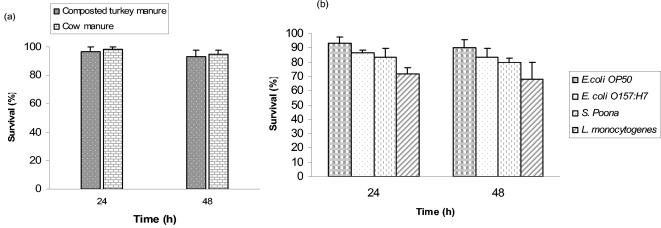
Manure and compost-amended soils are often used to grow vegetables and fruits intended to be eaten raw. Since application of manure and compost is believed to stimulate microbial activity and affect nematode populations (5), the ability of Diploscapter sp. strain LKC25 to survive in cow manure and composted turkey manure was investigated. Changes in populations of Diploscapter sp. strain LKC25 inoculated into fresh cow manure and composted turkey manure were determined 24 and 48 h after inoculation. A high percentage (>90%) of the worms remained viable in cow manure 48 h after inoculation (Fig. 3a). The optimum temperature for growth of Diploscapter sp. strain LKC25 is ca. 30°C, compared to 20°C for C. elegans.
FIG. 3.
Survival of Diploscapter sp. strain LKC25 in cow manure and composted turkey manure (a) and in soil amended with composted turkey manure inoculated with E. coli OP50, E. coli O157:H7, S. enterica serotype Poona, or L. monocytogenes (b).
The recognized tolerance of Diploscapter sp. strain LKC25 to elevated temperature and its presence in compost (12) prompted an investigation to determine its ability to survive in composted turkey manure. Diploscapter sp. strain LKC25 was inoculated into composted turkey manure and held for up to 48 h at 30°C before analyzing to determine changes in the number of viable worms. A high percentage (>90%) of the population survived (Fig. 3a).
The survival of Diploscapter sp. strain LKC25 in soil amended with composted turkey manure and inoculated with pathogens or E. coli OP50 was determined. The presence of test bacteria did not markedly alter survival of Diploscapter sp. strain LKC25 in compost-amended soil, although higher survival of the worm was observed in amended soil containing E. coli OP50 compared to survival in the presence of pathogens (Fig. 3b). The lowest percentage of Diploscapter sp. strain LKC25 survived in amended soil inoculated with L. monocytogenes.
Attraction of Diploscapter sp. strain LKC25 to pathogenic bacteria.
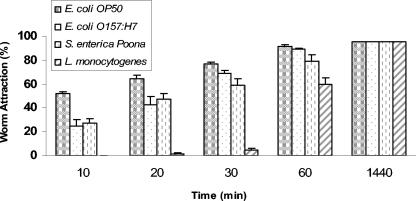
Once the ability of Diploscapter sp. strain LKC25 to survive and grow using food-borne pathogens as nutrient sources was demonstrated, its attraction to the same pathogens was evaluated. Worms were attracted to E. coli O157:H7, S. enterica serotype Poona, and L. monocytogenes as well as to nonpathogenic E. coli OP50 on TSAN (Fig. 4). More than 50% of the worms initially placed 1 cm away from the sites of inoculation of E. coli OP50, and all pathogens migrated to the sites within 60 min. At 24 h, 95% of the worms migrated to colonies formed at sites of inoculation. Worms were more slowly attracted to pathogens than to E. coli OP50, which could be due to worms being conditioned for this food source. They were markedly less attracted to L. monocytogenes than to E. coli O157:H7 or S. enterica serotype Poona during the first 60 min of incubation. Listeria was the only gram-positive bacterium used in this study and may have factors characteristic of this bacterial group that repel worms.
FIG. 4.
Attraction of Diploscapter sp. strain LKC25 to E. coli OP50, E. coli O157:H7, S. enterica serotype Poona, and L. monocytogenes on TSAN.
Studies have shown that free-living nematodes such as C. elegans are attracted to bacteria over distances of several centimeters (8). Since most free-living nematode activity occurs within the top 5 cm of soil, attraction of Diploscapter sp. strain LKC25 to pathogenic bacteria (at a distance >1 cm) that may enter the soil from sources such as manure, runoff water, or irrigation water is plausible. We attempted to assess attraction over greater lengths by placing the worms at a distance of approximately 5 cm from each strain of bacterium over 60 min, followed by a 24-h observation. With these studies only one to three worms (out of 20 to 25) were found in bacterial colonies, with the majority remaining closer to the initial area of placement. Compared to C. elegans, which is approximately three times greater in length, Diploscapter sp. strain LKC25 was found to be more restricted in movement.
Shedding of pathogenic bacteria.
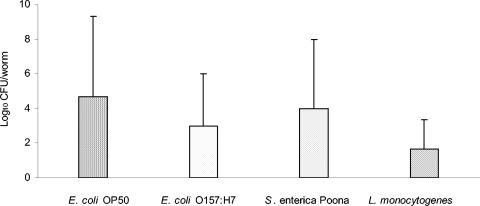
Shedding of bacteria that were in and on Diploscapter sp. strain LKC25 that had fed on food-borne pathogens or E. coli OP50 for 24-h in soil amended with composted turkey manure was investigated. Worms shed all four bacteria on TSAN during a subsequent 24-h incubation period (Fig. 5). There were no significant differences in the number of each test bacterium shed on the TSAN, indicating that shedding characteristics were not influenced by the genus or strain of the bacteria.
FIG. 5.
Shedding of pathogenic bacteria and E. coli OP50 by Diploscapter sp. strain LKC25 on TSAN within 24 h after exposure to bacteria inoculated into soil amended with composted turkey manure. Values (log10 CFU/worm) are means of the number of colonies formed on TSAN by bacteria shed by Diploscapter sp. strain LKC25 during the 24-h incubation period after removal from inoculated compost-amended soil.
In summary, the results show that Diploscapter sp. strain LKC25 may lack the ability to sense the presence of bacteria at distances greater than 1 cm. Previous studies indicate that C. elegans migrates to bacteria at distances greater than 1 cm (3, 10). Environmental vectors are among the primary factors known to cause contamination of produce with food-borne pathogens, and the potential for preharvest contamination of fruits and vegetables by bacterivorous nematodes may be among these factors. The results indicate that Diploscapter sp. strain LKC25 ingests and passes viable food-borne pathogenic bacteria. Our observations should serve as a foundation for more extensive research to determine the role of Diploscapter sp. strain LKC25 as one of several free-living nematodes that may impact the level of microbiological safety of preharvest fresh fruits and vegetables. Future work should assess a number of issues, including the effectiveness of sanitizers in killing food-borne pathogens ingested by Diploscapter sp. strain LKC25. The attraction of Diploscapter spp. to fruit and vegetable tissues should be studied. Also, more information is needed on basic aspects of this nematode's biology, including pH tolerance and methods for establishing synchronized cultures.
Acknowledgments
This research was funded in part by the United States Department of Agriculture, Agricultural Research Service, National Alliance for Food Safety and Security, Beltsville, MD. The USDA-ARS supplied the composted turkey manure used in this study. Mention of trade names of commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
We thank Eric Erbe and Charles Murphy of the USDA-ARS, Soybean Genomics and Improvement Laboratory, Beltsville, MD, for assistance with electron microscopy, and Sharon Ochs of the USDA-ARS Nematology Laboratory, Beltsville, MD, for image editing.
REFERENCES
- 1.Aballay, A., and F. M. Ausubel. 2002. Caenorhabditis elegans as a host for the study of host-pathogen interaction. Curr. Opin. Microbiol. 5:97-101. [DOI] [PubMed] [Google Scholar]
- 2.American Society for Testing and Materials. 2001. Standard guide for conducting laboratory soil toxicity tests with the nematode Caenorhabditis elegans. E 2172-01. Annual Book of ASTM Standards, vol. 11.05. American Society for Testing and Materials, West Conshohocken, Pa.
- 3.Anderson, G. L., K. N. Caldwell, L. R. Beuchat, and P. L. Williams. 2003. Interactions of a free-living soil nematode, Caenorhabditis elegans, with surrogates of food-borne pathogenic bacteria. J. Food Prot. 66:1543-1549. [DOI] [PubMed] [Google Scholar]
- 4.Andrassy, I. 1993. A taxonomic review of the suborder Rhabditina (Nematoda: Secernetia). Orstrom, Paris, France.
- 5.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 6.Beuchat, L. R., and J. H. Ryu. 1997. Produce handling and processing practices. Emerg. Infect. Dis. 3:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaxter, M. L., P. ed. Ley, J. R. Garvey, L. X. Liu, P. Scheldeman, A. Vierstraete, J. R. Vanfleteren, L. Y. Makey, M. Dorris, L. M. Frisse, J. T. Vida, and W. K. Thomas. 1998. A molecular evolutionary framework for the phylum Nematoda. Nature 392:71-74. [DOI] [PubMed] [Google Scholar]
- 8.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck, J. W., R. R. Walcott, and L. R. Beuchat. 21 January 2003, posting date. Recent trends in microbiological safety of fruits and vegetables. Plant Health Progress http://www.plantmanagementnetwork.org/sub/php/review/2003/safety/. [Online.]
- 10.Caldwell, K. N., G. L. Anderson, P. L. Williams, and L. R. Beuchat. 2003. Attraction of a free-living nematode, Caenorhabditis elegans, to food-borne pathogenic bacteria, and its potential as a vector of Salmonella Poona to preharvest contamination of cantaloupe. J. Food Prot. 66:1964-1971. [DOI] [PubMed] [Google Scholar]
- 11.Carta, L. K. 2000. Bacterial-feeding nematode growth and preference for biocontrol isolates of the bacterium Burkholderia cepacia. J. Nematol. 32:362-369. [PMC free article] [PubMed] [Google Scholar]
- 12.Lemzina, L. V., and V. G. Gagarin. 1994. New species of free-living nematodes from thermal waters in Kyrghystan. Zoosystematic-Rossica 3:19-21. [Google Scholar]
- 13.Mead, P. S., L. Slutsker, V. Dietz, L. F. McGaig, J. S. Bresee, C. Sharpiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi, R. M. 1998. Carinoscapter cornutus gen. n., sp. n. Diploscapter striatus sp. n. and D. angolaensis sp. n. (Rhabditida: Diploscapteridae). Int. J. Nematol. 8:61-67. [Google Scholar]
- 15.Wergin, W. P., and A. R. Stone. 1981. Techniques for preparation and examination of plant-parasitic nematodes in the scanning electron microscope. Scanning Electron Microsc. 3:169-176. [Google Scholar]
- 16.Williams, P. L., and D. B. Dusenbery. 1990. Aquatic toxicity testing using the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 9:1285-1290. [PubMed] [Google Scholar]