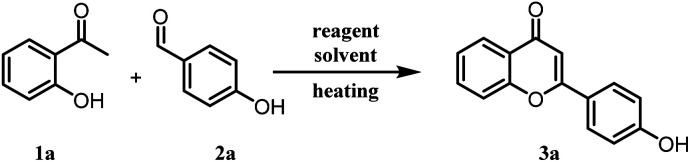
Optimization of reaction conditions for the synthesis of chromonesa.
| |||||
|---|---|---|---|---|---|
| Entry | Reagents (equiv.) | Solvents | Temp. (°C) | Time (h) | Yieldb (%) |
| 1 | I2 (0.5) | Ethanol | 80 | 72 | 7 |
| 2 | I2 (1.0) | Ethanol | 80 | 72 | 14 |
| 3 | I2 (1.0) | EG | 140 | 72 | nd |
| 4 | I2 (1.0) | Acetonitrile | 80 | 72 | nd |
| 5 | I2 (1.0) | Toluene | 80 | 72 | nd |
| 6 | I2 (1.0) | EtOAc | 80 | 72 | nd |
| 7 | I2 (1.0) | DMF | 140 | 72 | nd |
| 8 | I2 (1.0) | NMP | 140 | 72 | nd |
| 9 | I2 (1.0) | DMSO | 140 | 72 | nd |
| 10 | I2 (1.0) | Xylene | 140 | 72 | nd |
| 11 | I 2 (1.0) | PEG-400 | 140 | 7 | 84 |
| 12 | I2 (0.1) | PEG-400 | 140 | 72 | 13 |
| 13 | I2 (0.3) | PEG-400 | 140 | 72 | 39 |
| 14 | I2 (0.5) | PEG-400 | 140 | 72 | 58 |
| 15 | NaI (1.0) | PEG-400 | 140 | 72 | nd |
| 16 | CuI (1.0) | PEG-400 | 140 | 72 | nd |
| 17 | KI (1.0) | PEG-400 | 140 | 72 | 8 |
| 18 | I2 + KI (1.0) | DMF | 140 | 72 | 28 |
| 19 | I2 + KI (1.0) | Toluene | 80 | 72 | 32 |
| 20 | I2 + KI (1.0) | Ethanol | 80 | 72 | 22 |
| 21 | PhI(OAc)2 | PEG-400 | 140 | 48 | nd |
Reagents and conditions: different reagents were screened as given in Table 1 with varied temp. and equivalencies.
Isolated yield, nd - not detected.
