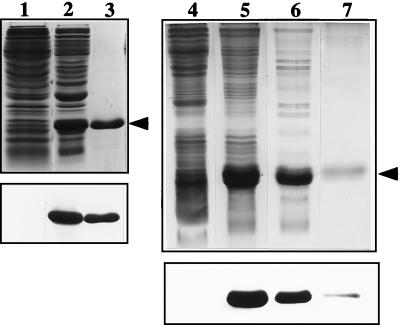
FIG. 3.
Purification of recombinant Bm-MIF. Lanes 1 to 3, pRSETB. Protein extracts from induced bacteria carrying pRSETB with no insert (lane 1) and from induced bacteria carrying pRSETB-Bm-mif (lane 2) are shown. The induced extract was passed over a nickel affinity column, and the 16.5-kDa His-tagged Bm-MIF (Bm-MIF-His) fusion protein was eluted with imidazole (lane 3). Extracts were separated on a 15% polyacrylamide gel under reducing conditions and visualized with Coommassie blue staining (top). The proteins from an identical gel were transferred to a nitrocellulose membrane and immunostained with anti-Bm-MIF-His (bottom). Lanes 4 to 7, pET11b. Protein extracts from induced bacteria carrying pET11b with no insert (lane 4) and from induced bacteria carrying the pET11b-Bm-mif construct (lane 5) are shown. The extracts of induced bacteria were passed over a MonoQ column (lane 6), and the flowthrough was placed on a butyl-Sepharose column. The 12.5-kDa Bm-MIF protein was eluted from the butyl-Sepharose with decreasing amounts of salt (lane 7). Proteins were separated on a 15% polyacrylamide gel under reducing conditions and visualized with silver staining (top). The proteins from an identical gel were transferred to a nitrocellulose membrane and immunostained with anti-Bm-MIF-His (bottom).