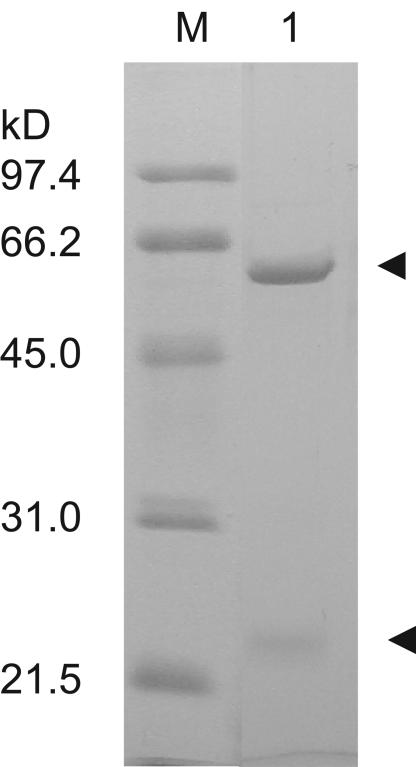
FIG. 1.
SDS-PAGE analysis of purified AhlM. The enzyme was purified from recombinant S. lividans, as described in Materials and Methods. The pooled active fractions were loaded on SDS-12% PAGE after Ni affinity chromatography. The arrowheads indicate the bands corresponding to two subunits of AhlM. Lane M, size marker; lane 1, purified AhlM.