Fig. 1 |. Model of the human S-OPA1 bound to membranes.
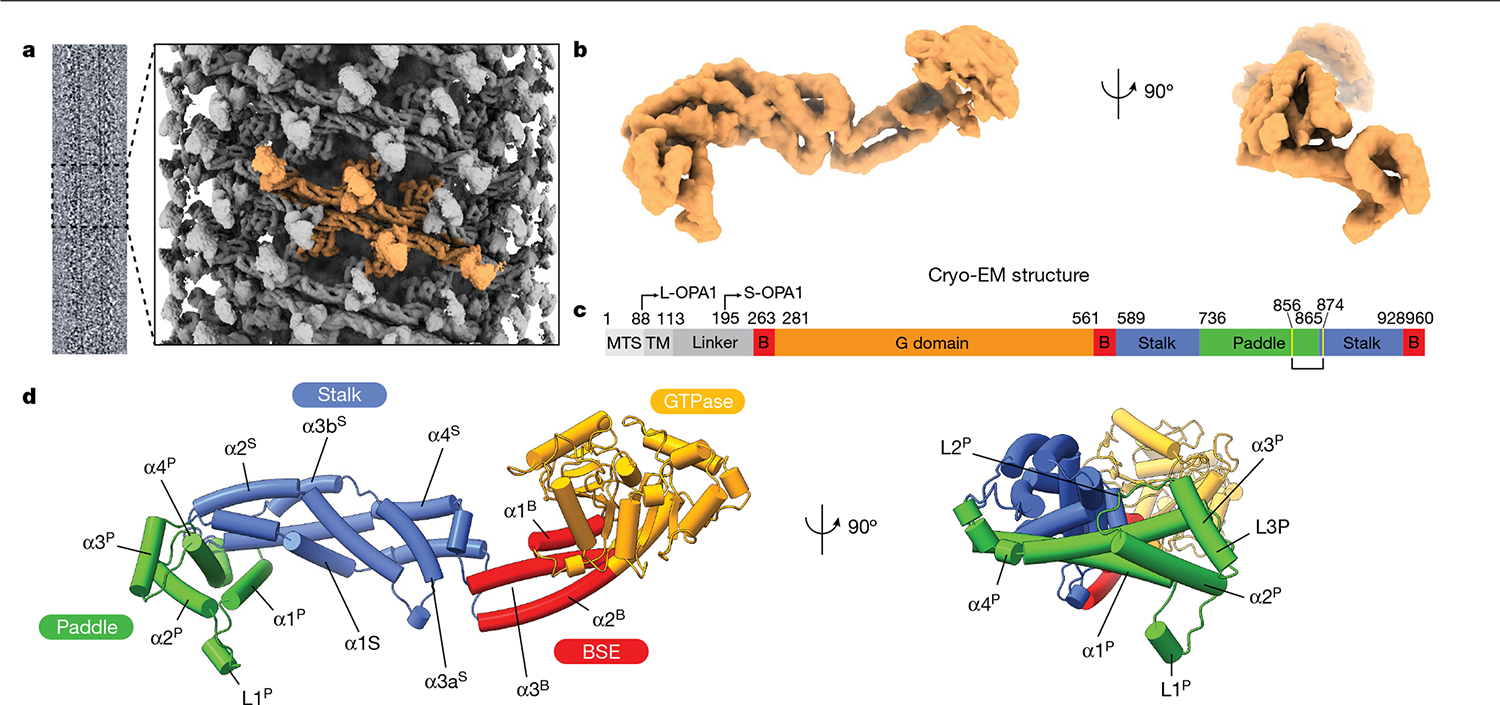
Lipid-binding triggers the formation of higher-order OPA1 assemblies. a, Cryo-EM image of the S-OPA1–membrane assembly and a side view of the cryo-EM density map of the membrane-bound S-OPA1 assembled in the presence of GMPPCP. Four S-OPA1 subunits forming a tetrameric assembly are coloured in orange. b, Side and back views of the molecular structure of the isolated S-OPA1 subunit. c, Schematic of the domain organization of full-length human OPA1. TM, transmembrane domain; B, BSE. L- and S-OPA1 describe the transmembrane-anchored long-form and proteolytically processed short form. Cysteine residues that form a disulfide bond are highlighted in yellow. d, Corresponding views of the structural model of the membrane-bound S-OPA1. Domains are coloured individually here and in all of the subsequent figures as in c. The GTPase, BSE and stalk domains are a conserved hallmark of the dynamin-related protein family. The GTPase domain consists of a central β-sheet surrounded by α-helices. The BSE was built of an extended three-helical bundle (α1B–α3B) that displays an amphipathic character, whereby the hydrophobic residues pack into a core and charged residues are accessible to the solvent. At the N terminus, the GTPase domain is connected to the stalk domain by the α1B of the BSE. The stalk domain of OPA1 consists of four antiparallel α-helices forming an extended bundle (α1S–α4S). The conformational changes in these domains are transmitted to the lipid-binding PD to reinforce its ability to remodel membranes.
