Extended Data Fig. 5 |. Human S-OPA1 map quality, model building, and structural comparison with the yeast Mgm1.
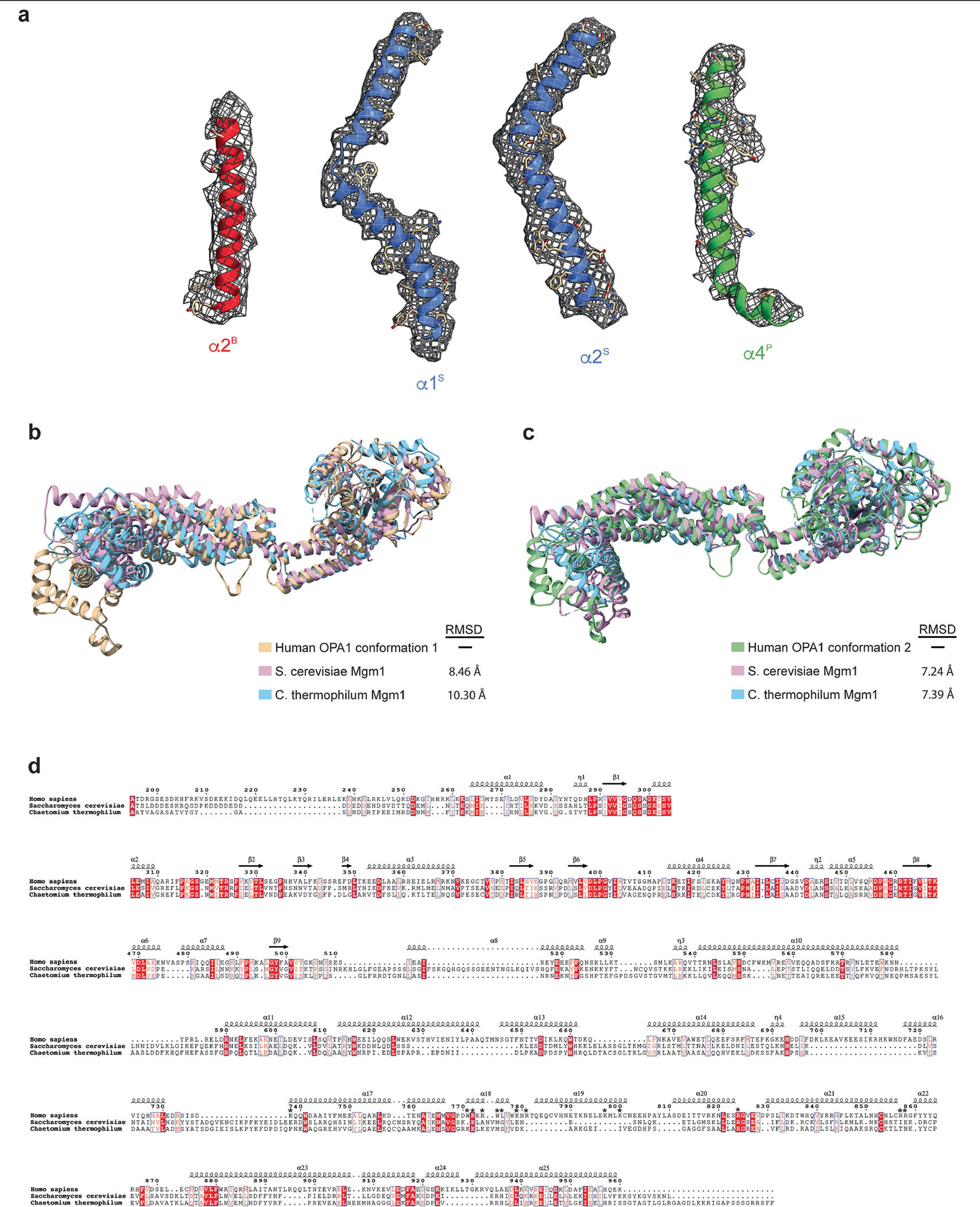
a, Isolated S-OPA1 monomer EM density from post-processed maps showing the quality of the map, build and fit. A single view of the cryo-EM density is depicted as a semi-transparent mesh and superimposed upon the model. Examples of model fit within B-factor sharpened cryo-EM density for the alpha-helices of S-OPA1 BSE (red), stalk (blue), paddle (green) are shown in the context of the atomic model with side chains are shown as sticks and the backbone as ribbons. b, A comparative analysis of the human S-OPA1 to yeast structures deposited in the PDB using topology independent comparison server CLICK revealed that the S-OPA1 structure displays a similar topology for the GTPase, BSE, and stalk domains; however, the paddle domain adopts a novel architecture with the addition of α3P helix, which facilitates the formation of interface 7. Structural comparison of membrane-proximal conformation of human S-OPA1 (beige) to S. cerevisiae (Pink, PDB ID: 6JSJ) and C. thermophilum (Light blue, PDB ID: 6QL4) Mgm1 crystal structures. Overall, the membrane-proximal conformation of human S-OPA1 and S. cerevisiae Mgm1 align with an r.m.s.d. of 8.46 Å, and human S-OPA1 and C. thermophilum Mgm1 align with an r.m.s.d. of 10.30 Å over all Cα atoms. c, Superimposition of membrane-distal conformation of human S-OPA1 (beige), S. cerevisiae (Pink), and C. thermophilum (Light blue) Mgm1 structures. Overall, the membrane-distal conformation of human S-OPA1 superimposes with S. cerevisiae Mgm1 and C. thermophilum Mgm1 with a rmsd of ~7.3 Å over all Cα atoms. d, Multiple sequence alignment of human S-OPA1, S. cerevisiae S-Mgm1, and C. thermophilum S-Mgm1. There is ~21% sequence conservation between human and yeast proteins. Most of the residues involved in binding to membranes are not conserved (highlighted with an asterisk).
