Abstract

Zinc (Zn) is a crucial element with remarkable significance in organic transformations. The profusion of harmless zinc salts in the Earth’s outer layer qualifies zinc as a noteworthy contender for inexpensive and eco-friendly reagents and catalysts. Recently, widely recognized uses of organo-Zn compounds in the field of organic synthesis have undergone extensive expansion toward asymmetric transformations. The ProPhenol ligand, a member of the chiral nitrogenous-crown family, exhibits the spontaneous formation of a dual-metal complex when reacted with alkyl metal (R–M) reagents, e.g., ZnEt2. The afforded Zn complex possesses two active sites, one Lewis acid and the other Brønsted base, thereby facilitating the activation of nucleophiles and electrophiles simultaneously within the same chiral pocket. In this comprehensive analysis, we provide a thorough account of the advancement and synthetic potential of these diverse catalysts in organic synthesis, while emphasizing the reactivity and selectivities, i.e., dr and ee due to the design/structure of the ligands employed.
Introduction
Zinc (30Zn) occupies the 24th spot in terms of abundance among the elements found in the Earth’s crust. It has five stable isotopes, out of which 64Zn is found most abundantly with 48.6% natural abundance.1 Due to the inherent elemental properties, Zn(II) salts find extensive application as Lewis acids in various nucleophilic addition reactions. These include the Aldol reaction, the Mannich reaction, the Henry reaction, and the Michael addition reaction. Many efforts have been dedicated to applying these approaches for the construction of new carbon–carbon bonds or carbon quaternary stereocenters. The majority of these processes are believed to follow a mononuclear Zn(II) mediated pathway. Specifically, various compounds such as ZnCl2, ZnBr2, ZnI2, Zn(OTf)2, Et2Zn, and organozinc species generated in situ are suggested to involve mononuclear zinc complexes as vital intermediates in promoting nucleophilic additions.
The idea of dinuclear zinc pathways often gets overlooked because researchers are more familiar with the mononuclear pathway while considering the mechanistic study of organic transformations involving zinc. Surprisingly, dinuclear pathways are crucial, especially in enzyme chemistry.2 The zinc functions as a cocatalyst in the active site of various enzymes as an intrinsic cofactor, i.e., a carbonic anhydrase, which facilitates the transport of carbon dioxide and was the first metalloenzyme known.3 Currently, zinc-based enzymes belong to all the fundamental enzyme families, including oxidoreductase (dehydrogenase or reductase), transferase (transfer functional groups), hydrolase (involved in hydrolysis), lyase (cleavage of bonds without involving water), isomerase (rearrangement of molecular structures), and ligase or synthetase (involved in the formation of chemical bonds). This has led chemists to develop chiral catalytic systems involving zinc atoms that follow the dinuclear pathway.
Lately, there has been a growing demand for chiral compounds, particularly in industries such as agrochemicals and pharmaceuticals, due to the significant impact that enantiopurity has on the physical and biochemical properties of organic molecules. Consequently, research groups worldwide have placed considerable emphasis on the development of asymmetric catalytic transformations. These catalytic transformations aim to achieve not only exceptional diastereo-, enantio-, and regioselectivities but also high reaction rates within a practical period.4,5 Although at present many organic reactions are being conducted under catalytically controlled asymmetric environments, the majority of these transformations require the use of valuable and toxic transition metals such as palladium (Pd), iridium (Ir), rhodium (Rh), and ruthenium (Ru) as the catalytic system.6,7 Compared to these transition metals commonly employed in catalysis, zinc (Zn), copper (Cu), and iron (Fe) complexes afford the advantages of being inexpensive, easily available, and less toxic.8
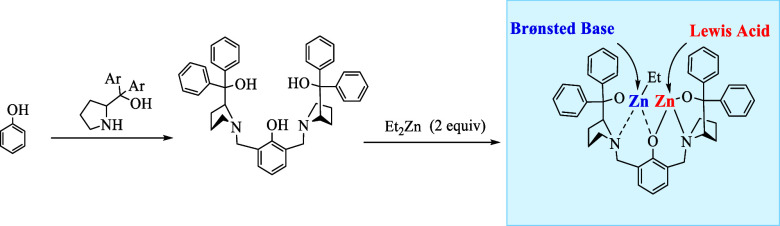
In 2000, Trost and co-workers developed ProPhenol, an aza-crown ligand derived from proline. The ligand upon reacting with an alkyl metal reagent like Et2Zn, autonomously affords a bimetallic Zn complex by deprotonating the three hydroxyl groups. The bimetallic complex contains two active sites: the Brønsted base site and the Lewis acid site (Scheme 1).
Scheme 1. Synthesis and Structure of the Bimetallic Zn-ProPhenol Complex.
The basic site functions via activating a pronucleophile by simple deprotonation. However, the acidic site functions by activating the electrophilic reactant by coordination with the metal. This activation of both reactants in the same chiral environment accounts for the highly effective bond formation in a stereoselective manner.9 The present report successfully outlines the advancements in the application of zinc binuclear catalysts in various asymmetric enantioselective transformations, including: Aldol reaction, Friedel–Crafts reaction, Mannich reaction, Michael addition reaction, N-alkylation of indol, 1–6-conjugated addition reaction, Aza-Henry reaction, and one-pot methodologies, i.e., domino and tandem reactions.
Asymmetric Aldol Reaction
Among the classic reactions for carbon–carbon (C–C) bond formation is an aldol reaction, which delivers a variety of β-hydroxy carbonyl compounds or α,β-unsaturated carbonyl compounds. The aldol reactions can be catalyzed by employing acidic conditions, basic conditions, metal–catalytic complexes, or enzymes.10
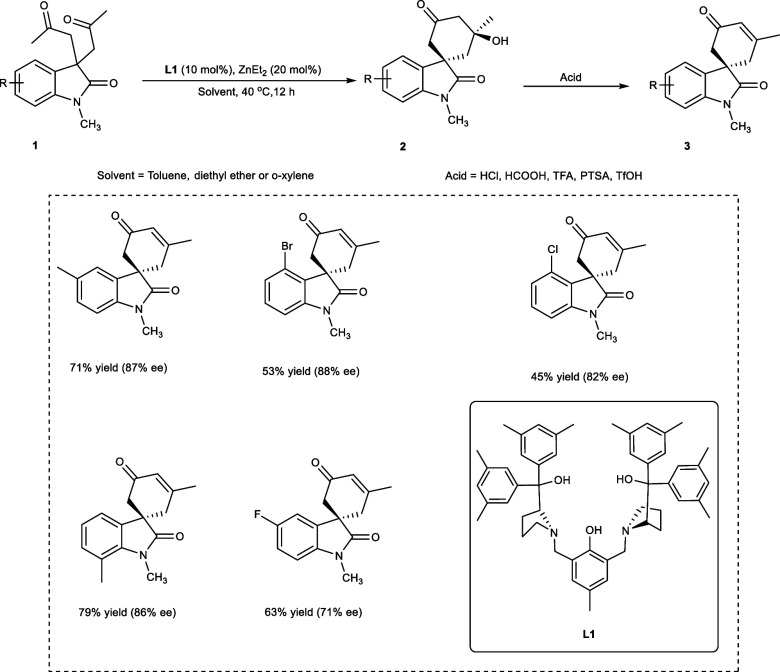
In 2016, Zhang et al. employed oxindole-derived diketone derivatives (1) as a starting substrate for an asymmetric desymmetrizing intramolecular aldol addition reaction/aldol condensation reaction affording spiro[cyclohexanone-oxindole] derivatives (2, 3) (Scheme 2).
Scheme 2. Synthesis of the Spiro[cyclohexanone-oxindole] Related Compounds Employing a Dinuclear Zinc Catalytic System.
The Trost’s bis-ProPhenol binuclear zinc complex of ligand (S,S)-L1 has been employed as a catalytic partner affording moderate to good enantioselectivities of the desired products. At room temperature, (S,S)-L1, a bis-ProPhenol ligand, is the most effective choice alongside 30 mg of the 4 Å molecular sieves (MS) in the catalytic solution, yielding 80% of the required products with 1:10 diastereoselectivity and 97% enantioselectivity.
The Brønsted acids were added for the dehydration of the aldol products. The reaction goes very well, giving 85–90% yield of the desired products in toluene, diethyl ether, or o-xylene with 94–97% ee values and 1:91:10 diastereoselectivity (dr) ratios. However, upon elevating the temperature of the reaction to 40 °C, yield drops to 68%, though the products are obtained with excellent stereoselectivities, i.e., 1:20 dr and >99% ee. The substrates having electron-rich substituents (methyl, methoxy) on the oxindole give aldol product in 50–81% yields, with varying enantioselectivities (82–93%) and diastereoselectivities (1:31:20). On the contrary, halogen (X = F, Cl, Br) on the oxindole backbones negatively affects the reaction, giving the desired products with 63–88% ees in 45–70% yield.11
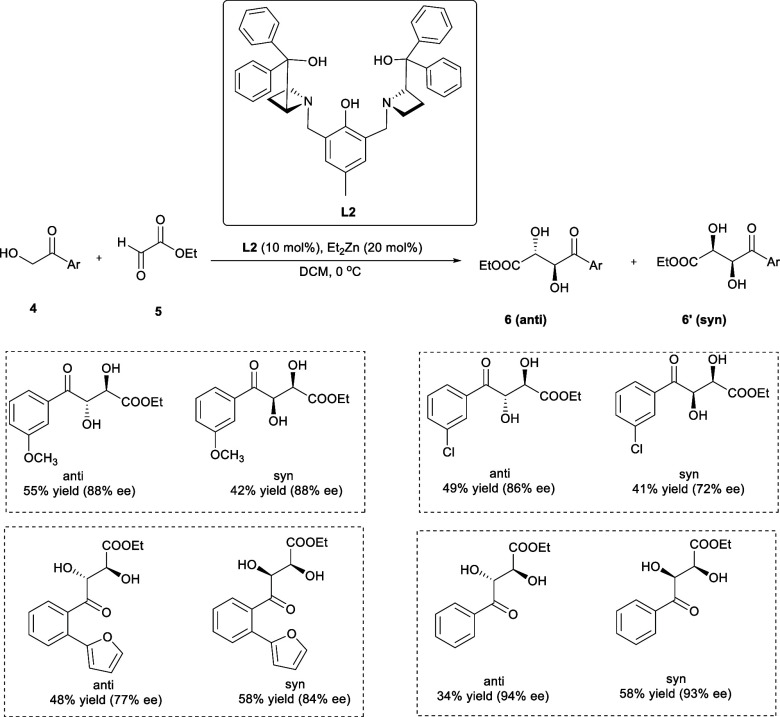
The α,β-dihydroxy ester-related compounds are significant pharmacophores and asymmetric building blocks that serve as the basis of bioactive polyketide natural and synthetic drugs.12 In 2019, Zhang et al. synthesized anti- as well syn-α,β-dihydroxy γ-keto ester derivatives (6, 6′) by the asymmetric aldol addition process involving 2-hydroxy-acetophenone (4) and ethyl glyoxylate (5) catalyzed by binuclear zinc (Zn)-AzePhenol catalyst (Scheme 3).
Scheme 3. Synthesis of the α,β-Dihydroxy γ-Keto Ester Derivatives by the Asymmetric Aldol Addition Reaction.
The solvent screening verified that dichloromethane (CH2Cl2) was the best choice to achieve excellent enantioselectivity. Also, the enantioselectivity of desired products has been improved up to 91% by lowering the temperature to 0 °C. The 2-hydroxy-acetophenone substituted with the p-methyl group has resulted in the high enantioselectivity (96% ee) of the corresponding syn-product in moderate yield (33%), while the anti-product has been obtained in higher yield (57%) with lower enantioselectivity (93% ee). The substitution with the p-methoxy group displayed excellent enantioselectivity (96% ee) but a lower yield (41%). Furthermore, the 2-hydroxy-acetophenone substituted with o-methyl or o-methoxy groups have shown outstanding enantioselectivities, while electron-deficient substituents, particularly the nitro (−NO2) group, have diminished the enantioselectivities. The reaction is also very well tolerated in the case of heteroaromatic compounds bearing electron-rich substituents.
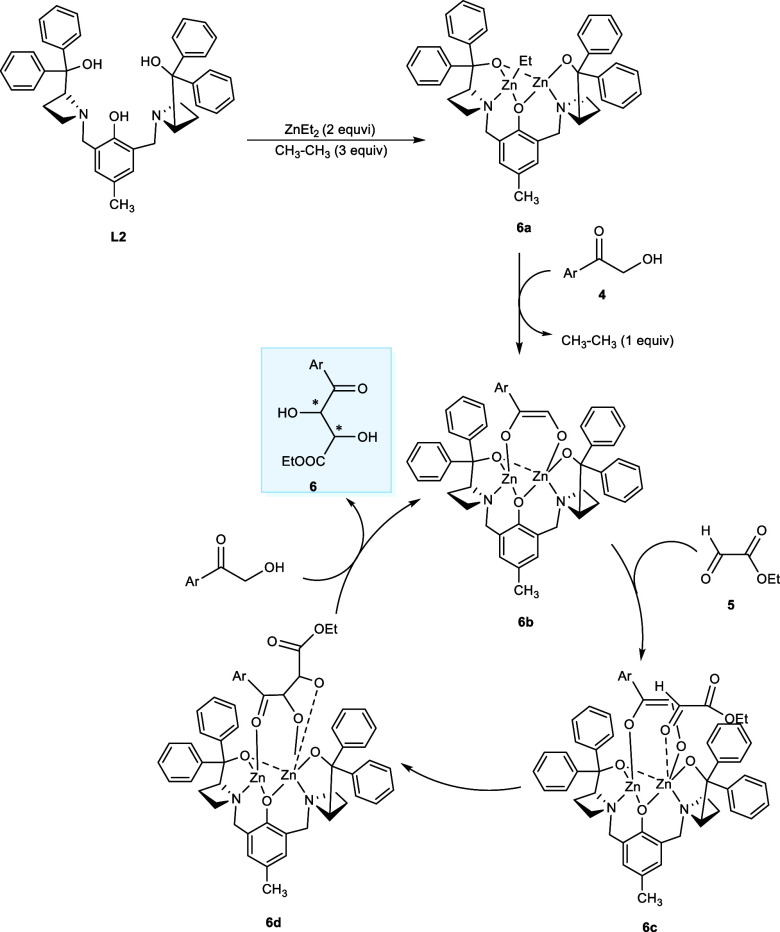
The mechanism of the reaction involves the deprotonation of 2-hydroxyacetophenone (4) by a dinuclear Zn-catalytic system (6a) affording the nuclear Zn-AzePhenol complex (6b). In the next step, the ethyl glyoxylate (5) combines with Lewis acidic Zn nucleus affording the intermediate complex (6c) followed by the aldol addition reaction to give (6d). Lastly, proton (H+) exchange with the coming nucleophile takes place, giving the desired product, and one catalytic cycle is completed. The highly reactive ethyl glyoxylate would not bind strictly to the Zn-atom, leading to a diastereoisomeric mixture in the aldol addition reaction (Scheme 4).13
Scheme 4. Asymmetrical Zn-Catalyzed Cycle for the Aldol Addition Reaction.
Asymmetric Mannich Reaction
Modern synthetic chemists are confronted with the challenge of achieving fast, selective, and atom-economical reactions to synthesize complex organic compounds from simple and readily accessible starting substrates.5 The presence of quaternary stereocenters is common in natural compounds, but their synthesis, especially through catalytic enantioselective means remains challenging.14 The Mannich transformation is considered to be among the efficient methods for forming carbon–carbon bonds and a range of asymmetric conversions, including a variety of Mannich transformations, are possible using Zn-ProPhenol ligands.15
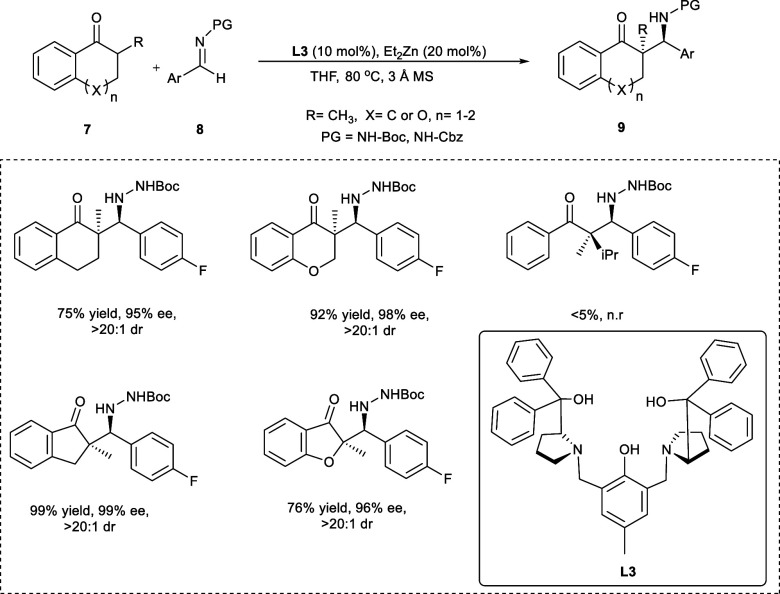
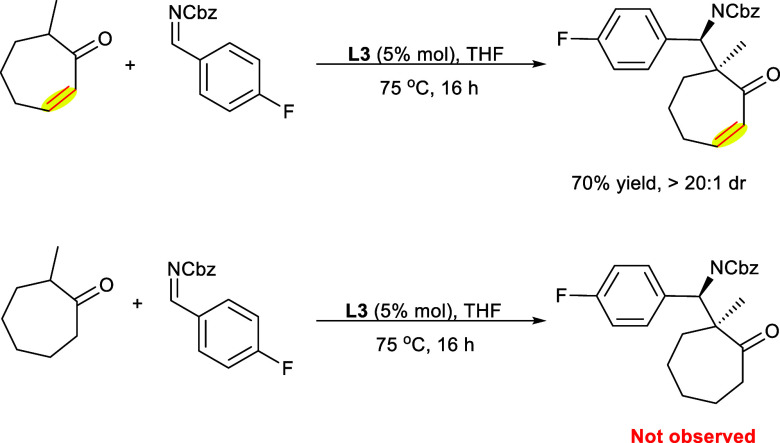
In 2016 Trost et al. reported a Mannich reaction catalyzed by a Zn-ProPhenol ligand using α-branched ketones as the starting material. The dinuclear catalysts are obtained by reacting the ProPhenol ligand from the aza-hemicrown ligand family with alkyl (R) metal reagents, e.g., diethylzinc (Et2Zn). The catalytic system Zn-ProPhenol has been highly effective for diastereoselective and enantioselective aldol addition reaction and Mannich reactions. The α-branched ketones (7) act as the nucleophile upon reacting with the Boc-protected derivative of aldimine (8) affording functionalized β-amino ketones (9) with every carbon as quaternary stereocenter utilizing Zn-ProPhenol ligand (R,R-L3) (Scheme 5). The reaction at 80 °C results in the end products with high selectivities in good yields. However, upon decreasing the temperature to 60 °C, the selectivities remain unaffected, decreasing the yields due to poor conversion. Both the electron-rich and -deficient groups have been compatible with heteroaromatic imines and aromatic rings during the reaction. The reaction also proceeded smoothly with alkyl imine and vinyl imine, providing the required products in high yields. Moreover, Cbz-protected imine derivatives showed similar efficiency, allowing orthogonal protective group techniques to be utilized.16
Scheme 5. Synthesis of the β-Amino Ketones Using Zn-ProPhenol Ligand.
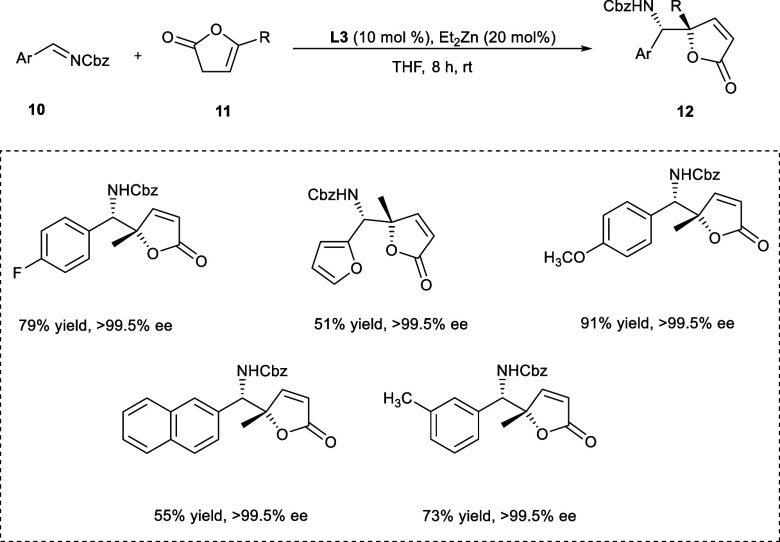
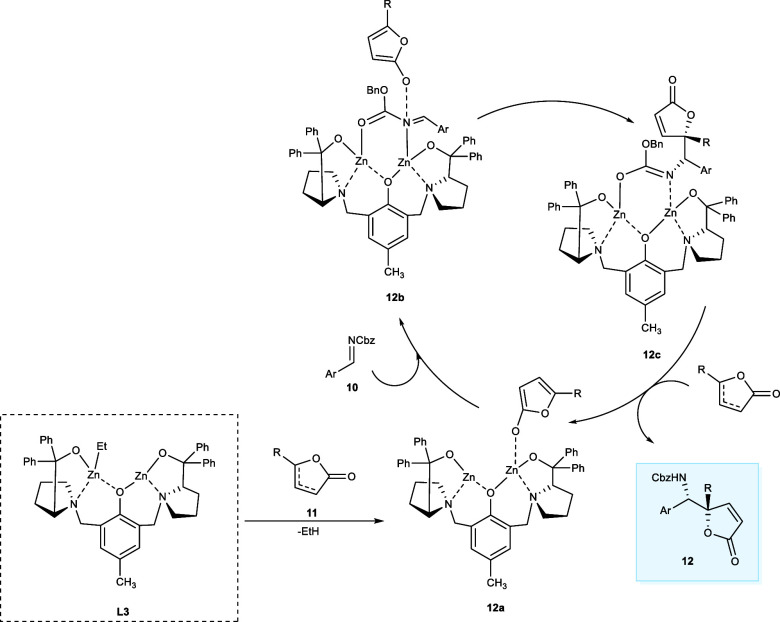
The butenolides containing nitrogen are found in several natural and medicinal compounds and are valuable synthetic intermediates.17 The GABA antagonists such as (−)-securinine and its isomers have been explored for their anticancer effects.18 In 2017, Trost et al. synthesized the tetrasubstituted vinylogous product (12) from imine derivatives (10) and α-angelica lactone (11) using an (R,R)-Zn-ProPhenol catalytic system (Scheme 6). The targeted products have been synthesized in good yield having diastereoselectivity up to >30:1 and excellent enantioselectivity, ranging from 97% to greater than 99.5%. According to the mechanistic studies, in the first step, zinc dienolate (12b) is formed by deprotonation of butenolide and coordination of dinuclear zinc–ligand complex (12a) obtained from ProPhenol-L3 and ZnEt2. In the next step, complex (12c) is produced by two-point attachment of imine, which leads to the binding of the butenolide to the si-face of the derivative of imine. The obtained diastereoselectivity of the product is because the shown conformation is preferred by butenolide minimizing the repulsion among the diphenylprolinol unit and ring structure (Scheme 7). The tetrahydrofuran (THF) has been the best choice of solvent to afford the maximum yield (up to 79%) of the desired products with 99.5% ee. Both the electron-donating and electron-withdrawing imine derivatives react effectively with α-angelica lactone affording the targeted products.19
Scheme 6. Mannich Reaction between Butenolides and Imines.
Scheme 7. Catalytic Cycle of the Mannich Transformation between Butenolides and Imines by Employing Binuclear Complex of (L3).
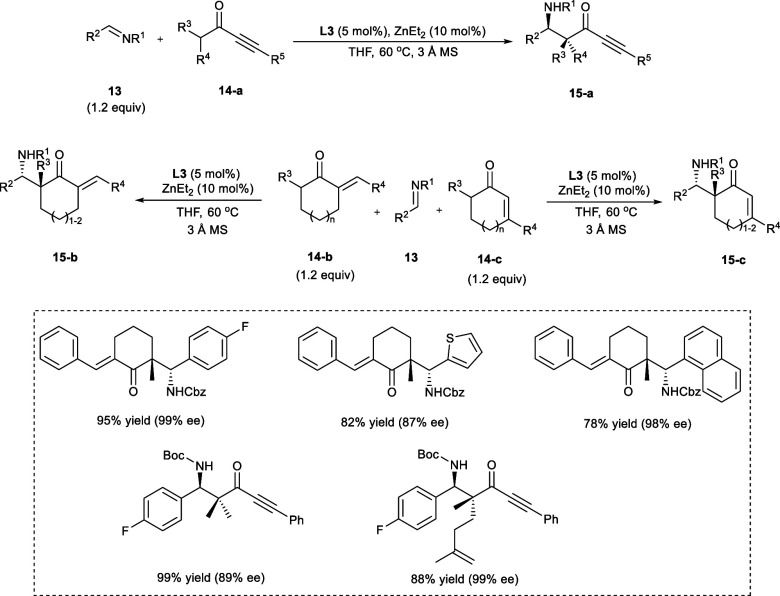
In 2019, Trost et al. carried the Mannich transformation between unsaturated α-branched ketones (14a–c) and N-carbamoyl imines (13), and synthesized β-aminoketones (15a–c) having both cyclic and aliphatic quaternary stereogenic centers (Scheme 8).
Scheme 8. Mannich Synthesis between Unsaturated α-Branched Ketone Derivatives and N-Carbamoyl Imines.
The incorporation of the carbon–carbon (C—C) double-bond adjacent to the carbonyl group (C=O) in α-branched ketones markedly improves the reactivity of the catalytic system, i.e., (R,R)-Zn-ProPhenol (Scheme 9). The imines with electron-rich, as well as electron-deficient aromatic ring, heteroaromatic, and vinyl imines, were tolerated well under the reaction conditions. The α-branched ketones having small groups at R3, such as methyl (−CH3), propargyl (HC≡C–CH2−) afford the products in high yield with remarkable stereoselectivities.20
Scheme 9. Effect of Unsaturation Added Adjacent to the Carbonyl in α,β-Branched Ketone Derivatives on the Reactivity of Zn-Catalyst.
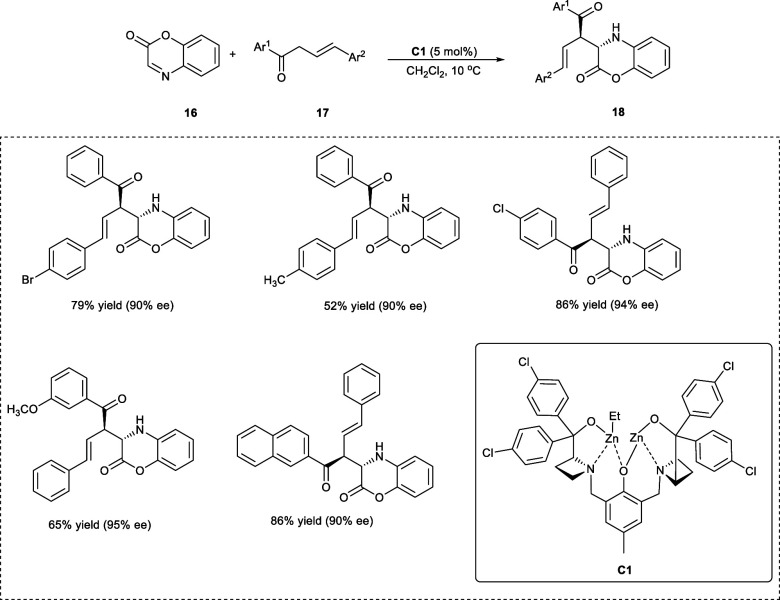
Pyridazinones and cyclic hydroxamates present in natural, as well as pharmaceutically active substances, are imperative intermediates for organic synthesis.21 Geng et al. in 2021 synthesized α-selective adduct (18) via a Mannich addition reaction between β,γ-unsaturated ketone derivatives (17) and benzoxazinone cyclic α-imino esters (16) (Scheme 10). The α-addition Mannich adducts bearing two consecutive 3° carbon stereocenters, have been obtained with high stereoselectivities, i.e., ee up to 99% and dr ratios of >20:1. The adducts could be employed as intermediates to synthesize multisubstituted tetrahydro pyridazinones and cyclocanalines. The reaction went smoothly upon the incorporation of both electron-donating (−CH3, −OCH3) and electron-withdrawing groups (X = −F, −Cl, −Br) at 4-position on the Ar2 of the β,γ-unsaturated ketone derivatives.22
Scheme 10. α-Selective Addition between β,γ-Unsaturated Ketone Derivatives and Benzoxazinone Cyclic α-Imino Esters.
Asymmetric Friedel–Crafts Alkylation Reaction
Chemists have been intrigued by the chemical transformation and biological potentials of nonproteinogenic amino acids. In many of the organic synthesis, 3-indolyl glycine compounds are employed as building blocks and essential synthetic intermediates.23
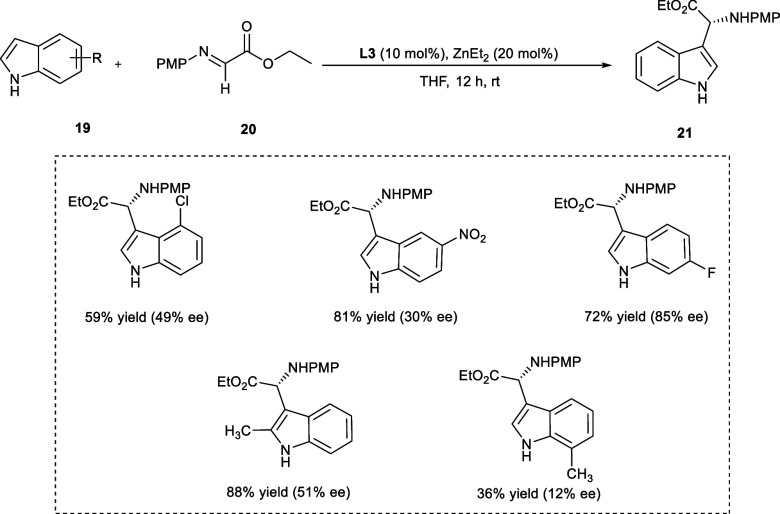
Wang et al. synthesized 3-indolyl-amino esters (21) via Friedel–Crafts asymmetrical alkylation from indole derivatives (19) and ethyl glyoxylate imine (20) by employing a dinuclear zinc catalytic system (Scheme 11).
Scheme 11. Synthesis of 3-Indolylglycine Derivatives by Asymmetric Friedel–Crafts Alkylation Using Zn-catalyst.
Under mild reaction circumstances, a variety of 3-indolyl glycine compounds have been afforded in medium to excellent yield with exceptional ee (up to >99%) upon only 10 mol % Zn-catalyst loading. The best results of the stereoselective Friedel–Crafts reaction were obtained using THF as a solvent for 12 h at room temperature. The indole derivatives having electron-releasing groups at the 4-position afford the targeted products with remarkable enantioselectivities (ee) of 91–94%. However, the reactivity of indoles has become sluggish upon introducing electron-withdrawing groups, e.g., X = −Cl, −F, affording good enantioselectivities (ee) in the products. The enantioselectivities with 5-substituted and 6-substituted indole derivatives were increased upon decreasing the steric crowding of substituted groups. The imine derivatives have shown high reactivity toward the electron-donating indoles, with excellent yields of the targeted products.
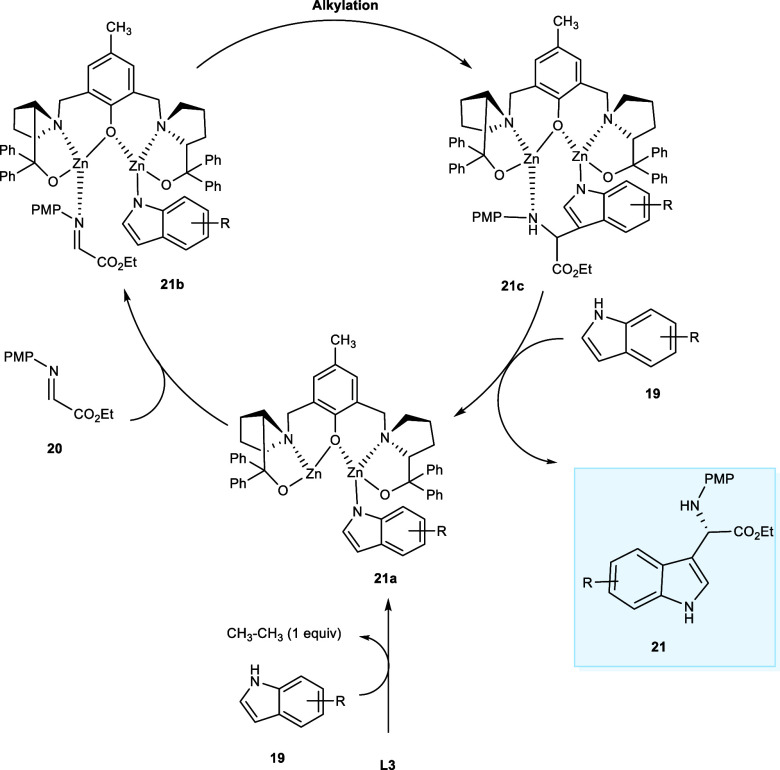
The reaction commences with the deprotonation of indole (19) liberating a molecule of ethane, affording the intermediate (21a). After coordination of ethyl glyoxylate imine (20) with the other zinc atom, the intermediate (21b) is obtained. The indole derivatives preferentially attack the imine’s C=N bond, from the reface to afford the intermediate (21c). In the last step, the desired adduct (21) is eliminated after the H+ transfer takes place between the intermediate (21c) and another indole (19) molecule, and the catalytic cycle is repeated (Scheme 12).24
Scheme 12. Catalytic Mechanism of the Reaction Involving Indole Derivatives and Ethyl Glyoxylate Imine.
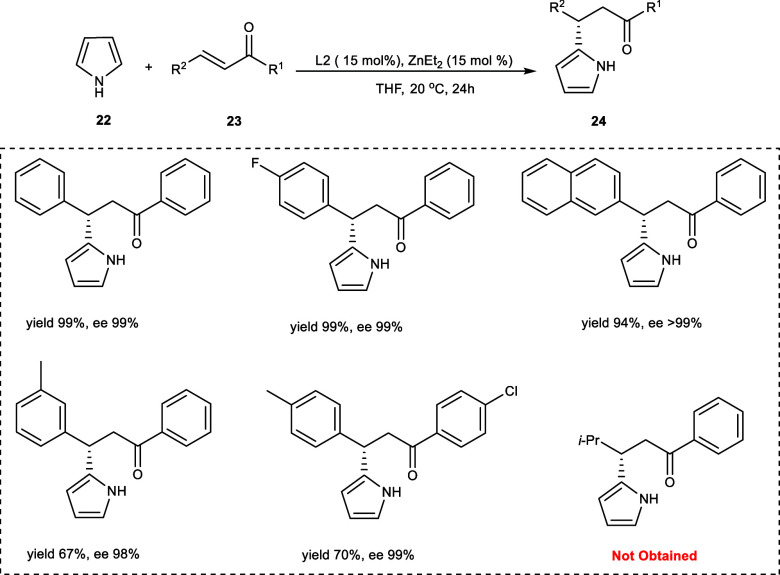
In 2014, Zhao et al. carried out an enantioselective catalytic Friedel–Crafts alkylation reaction between pyrrole (22) and nonchelating derivatives of chalcone (23). The (S,S)-L2 has been employed as the catalyst, and β-pyrrole-substituted dihydrochalcone derivatives (24) were obtained with excellent yield and enantioselectivity (Scheme 13). Upon screening the solvents toluene, CHCl3, CH2Cl2, and THF have been found to be a suitable choice for the reaction solvent. Moreover, the highest enantioselevities have been observed in the case of THF, and no reaction has been observed in the presence of 1,4-dioxane and CH3CN as solvent. The desired products have been obtained with excellent ee values regardless of the position or electronic nature of the substituent present on the aryl ring (R1). However, different effects on the reaction yields have been observed by the position and electronic nature of various substituents on the aryl ring (R1), and decrease in the yield has been observed in case of p-methyl and o-chloro. The electron-withdrawing substituents have been found more favorable in the reaction conditions on both (R1) and (R2) on the contrary decrease in yield was observed with m-methyl on (R2) without affecting the ee. The reaction has been a failure with alkyl-substituted α,β-unsaturated derivative of ketones. Upon treating the chalcone with N-methylpyrrole, N-benzoylpyrrole, and N-benzylpyrrole, no reaction has occurred, suggesting that the presence of the hydrogen atom on the nitrogen of the pyrrole ring is vital for the activation of reactants by the ligand.25
Scheme 13. Synthesis of β-Pyrrole-Substituted Dihydrochalcone Derivatives by Asymmetric Friedel–Crafts Alkylation using Zn-Catalyst.
Asymmetric Michael Addition Reaction
Recently, asymmetric organophosphates have become the focus of attention for chemists because of their potent biochemical actions, numerous applications as synthetic intermediates, and usage as a ligand for asymmetric metal-catalyzed reactions.26
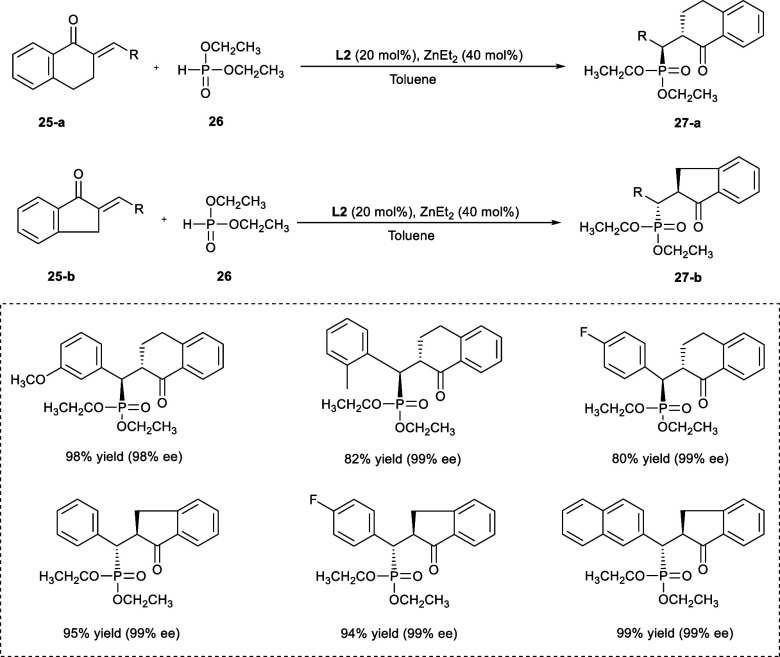
In 2018, Shao et al. employed a phospha-Michael type addition reaction between α,β-unsaturated benzocyclic ketone derivatives (25a–b) and diethyl phosphonate (26) to synthesis organophosphate compounds with the skeleton of 1-tetralones (27a) or 1-indanones (27b) (Scheme 14). The resulting targeted compounds were obtained in high yield with remarkable enantioselectivity, reaching a maximum of 99%. The benzylidene-1-tetralone derivatives having substituents at o-, m-, or p-positions on the β-phenyl ring undergo reaction smoothly, affording the required products in good yields with outstanding enantioselectives. The β-phenyl ring bearing the electron-donating substituents gives better results as compared to substrates with electron-withdrawing substituents. In the case of the substrates having electron-withdrawing groups such as −CF3 and −NO2 at the p-position, the enantioselectivity of desired products has been reduced slightly. Impressively, α-benzylidene-1-indanone derivatives have given the required products in remarkable yields with ee as high as 99% regardless of the positions and electronic properties of the substituents present.
Scheme 14. Synthesis of Organophosphate Compounds with the Skeleton of 1-Tetralones or 1-Indanones Using Zn-Catalyzed Phospha-Michael Reaction.
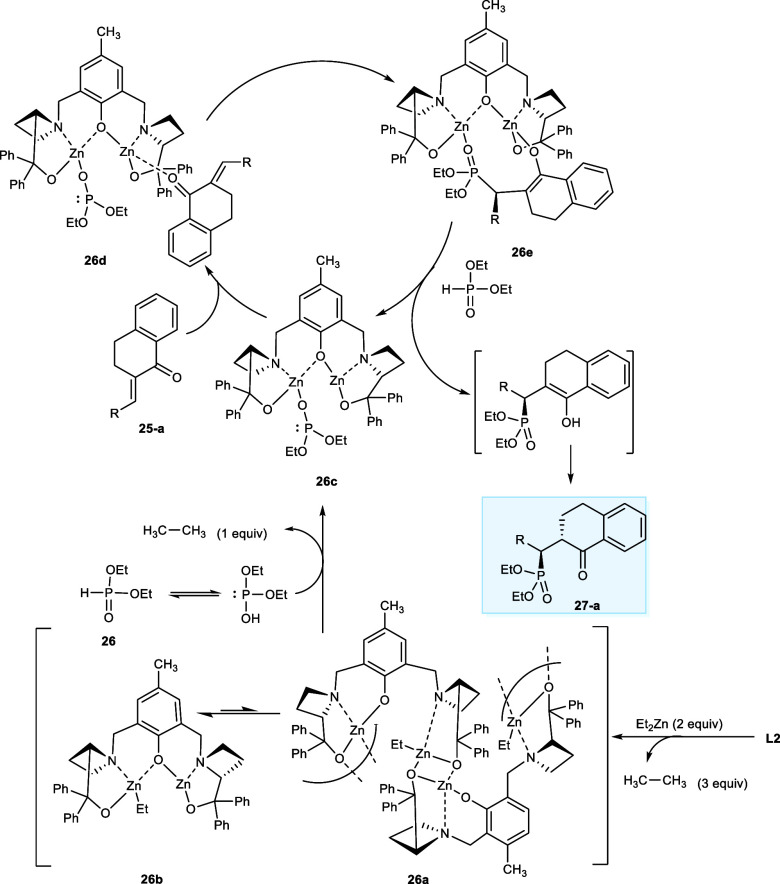
In the first step, ligand L2 reacts with diethyl zinc (ZnEt2), yielding a catalytically inactive dinuclear zinc atom complex (23a, 23b). The inactive complex is then converted to a catalytically active complex (23c) upon deprotonating the diethyl phosphonate (23) with the release of one ethane molecule. The α-benzylidene-1-tetralone (22-a) then combines with the other zinc metal atoms of the catalytic system to form an intermediate (23d), followed by the process of phosphoryl transfer to afford (23e). In the last step, a proton (H+) transfer occurs between (23e) and the diethyl phosphonate resulting in the final product, and the Zn-catalyzed cycle is repeated (Scheme 15).27
Scheme 15. Zn-Catalyzed Cycle for the Synthesis of Organophosphate Compounds with the Skeleton of 1-Tetralones or 1-Indanones.
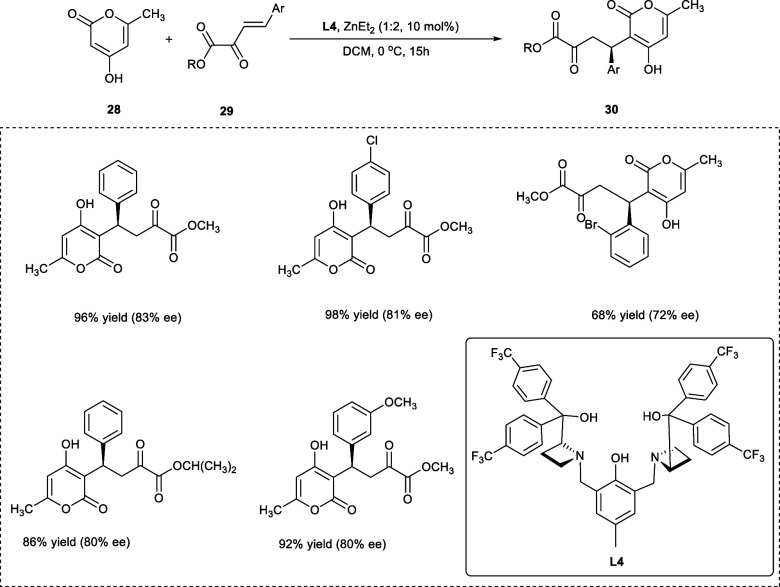
Pyranone and coumarin moieties are well-known structural scaffolds in a variety of bioactive chemicals. Their compounds are known as potential anti-HIV, anticoagulant, and antimalarial drugs.28 The optically active Michael 1,4-addition between 4-hydroxy pyrones (28) and 4-hydroxy coumarins (29) has been reported by Liu et al. to obtain β,γ-unsaturated α-keto esters (30) by using a CF3-substituted dinuclear Zn-AzePhenol catalyst (Scheme 16).
Scheme 16. Synthesis of the β,γ-Unsaturated α-Keto Ester Derivatives via Asymmetric Conjugated Addition.
The metallic catalytic strategy is efficient for the production of bioactive warfarin derivatives because of the large substrate scope, excellent enantioselectivity, and moderate reaction conditions. The required products have been obtained in excellent yield with an ee value of 83% under optimal reaction conditions in the presence of DCM at 0 °C. Impressively, the high yield with high enantioselectivity has also been obtained on lowering the Zn-catalyst loading from 20 to 10 mol %. In comparison to electron-withdrawing halogen substituents, the electron-donating substituents (e.g., methyl, methoxy) on the phenyl ring of the α-keto esters had a significant impact on both reactivity and asymmetric induction, because the electron-donating groups would promote the shifting of electronic cloud to carbonyl (C=O) group, making it less challenging to coordinate to stereogenic zinc catalyst.29
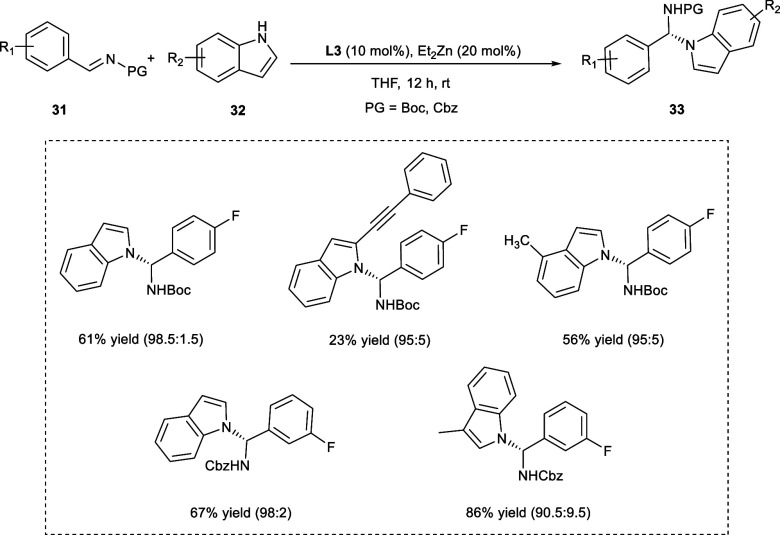
N-Alkylation Reaction
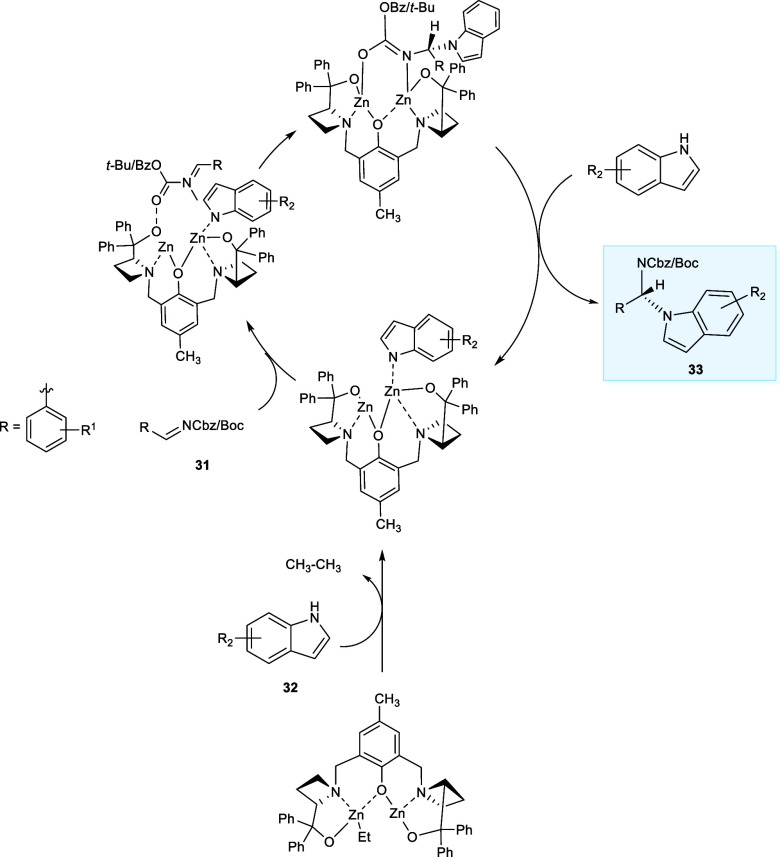
Indole as well as its derivatives have been studied extensively as therapeutic agents.30 In 2017, Trost et al. employed a zinc-ProPhenol dinuclear complex of ligand (L3) to catalyze the enantioselective N-alkylation of derivatives of indoles (32) with aldimines (31) to obtaine N-alkylated indole derivatives (33) (Scheme 17). The reaction affords satisfactory yields of N-alkylated compounds with enantioselectivity up to a 99.5:0.5 enantiomeric ratio (er), consequently opening the doors for their use in organic synthesis. The Zn-ProPhenol is responsible for the asymmetric alkylation at C3 when the Ts-protected imine has been employed as the amino-alkylating agent. However, the carbamate-protected derivatives of imines follow a chemical pathway that prevents C3-alkylation (Scheme 18).31
Scheme 17. Synthesis of N-Alkylated Indole Derivatives Using Zn-ProPhenol Dinuclear Complex.
Scheme 18. Binuclear Zn-Catalyzed Mechanism for N-Alkylation.
Asymmetric 1,6-Conjugate Addition Reaction
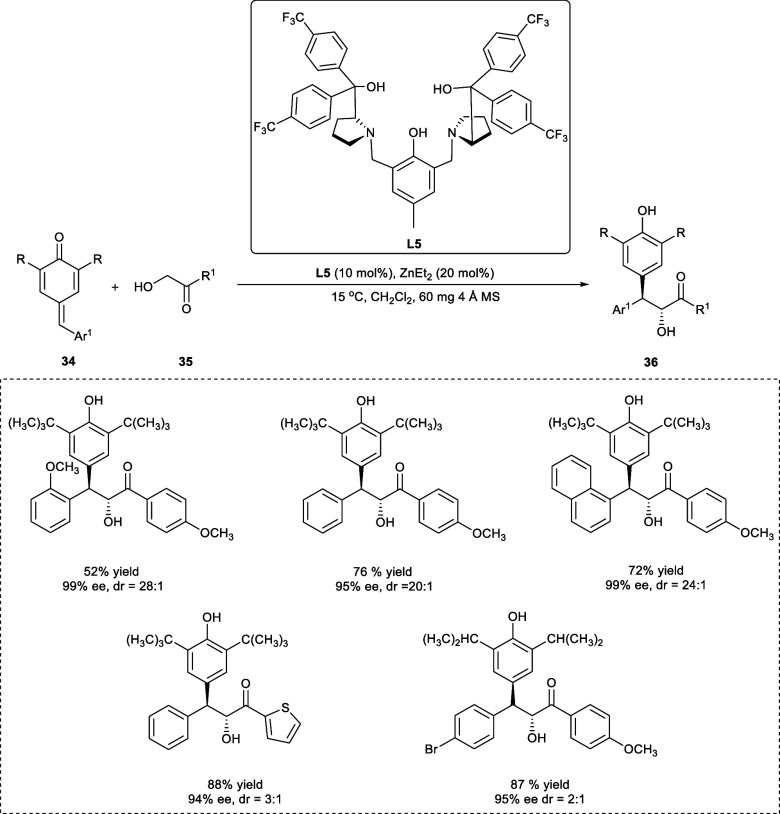
Stereoselective α-hydroxy ketones with two vicinal optically active sites are widely used in asymmetric synthesis as a chiral basic component, and they can be located in several organic products and medicine consequently, asymmetric ways to obtain such structural compounds have gotten much attention in present decades.32 In 2017, Gao et al. reported the production of chiral β,β-diaryl-α-hydroxy ketone derivatives (36) by an asymmetrical 1,6-conjugate addition reaction of α-hydroxy ketone (35) to p-quinone methides (34) (Scheme 19). The ligand (L5) outperformed the other employed ligands affording the major anticonformation diastereomers, with the dr value 11:5:1 and ee 98%. The higher diastereoselectivity has been achieved by introducing electron-donating groups (e.g., −OMe, −Me) in comparison to the electron-withdrawing groups at the p-position of the aromatic aryl ring. Moreover, the electron-withdrawing groups (e.g., fluoro, chloro, bromo) at the m-position of the aromatic aryl ring give the products with comparatively low dr ratios, i.e., 3.5:1–6.5:1. The substitution of electron-donating groups at the m-position has given the intended products with dr rations, 10:1–18:1. However, the desired compounds with dr rations, 13:1–99:1 has been obtained using p-QMs having different substituents at o-position of aromatic aryl ring.33
Scheme 19. Synthesis of β,β-Diaryl-α-hydroxy Ketone Derivatives via 1,6-Conjugated Addition Reaction Using Zn-Catalyst.
Asymmetric C–F Alkylation Reaction
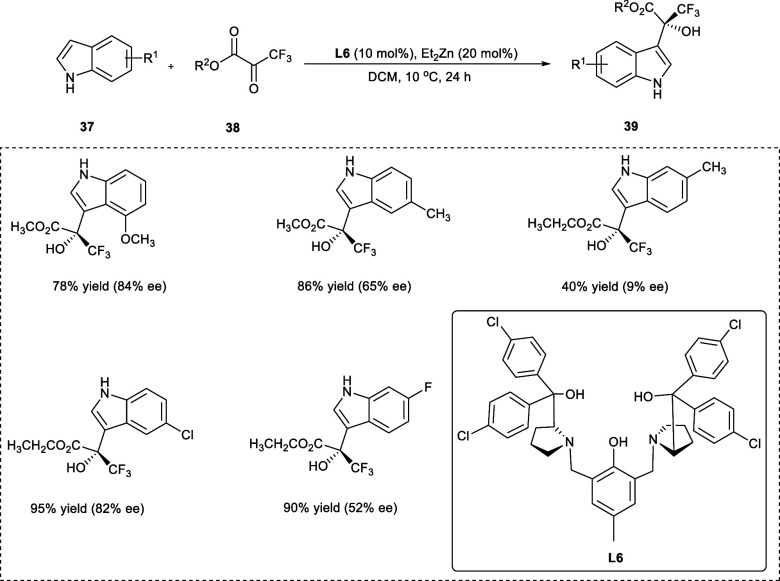
Fluorinated compounds have received a lot of interest in the past few years because of their unusual properties. The powerful electron-withdrawing effect as well as the absolute configuration on the −CF3 group result in the unusual biological and physical properties in chiral trifluoromethylated molecules, which are among the most prominent fluorine-containing chemical compounds.34 The chiral C–F alkylation of indoles (37) and trifluoromethylpyruvates (38) by dinuclear Zn-catalyst from ligand (S,S)-L6 yields useful asymmetric trifluoromethylated indoles (39) up to 95% with about 88% enantioselectivity (ee) (Scheme 20). The substrates having a strong electron-donating group (−OCH3) on the 4-position of the aryl ring performed well, giving 88% enantioselectivity. Conversely, the presence of a slightly weak electron-donating group (−Me) at the 5- or 6- position of the aryl ring has caused a decrease in the enantioselectivity i.e., 9–72% in the desired products. The presence of halogen groups (X= −F, −Cl, −Br) on the indoles has been helpful toward the catalytic Friedel–Crafts reaction, and much of the required products have been obtained with 80% enantioselectivity except for the 6-fluorinated end products. Strong electron-withdrawing groups, i.e., −CO2Me and −NO2, also affords equivalent compounds but with lower ee values.35
Scheme 20. Catalytic Asymmetric C–F Alkylation Reaction of Indoles and Trifluoromethyl Pyruvate.
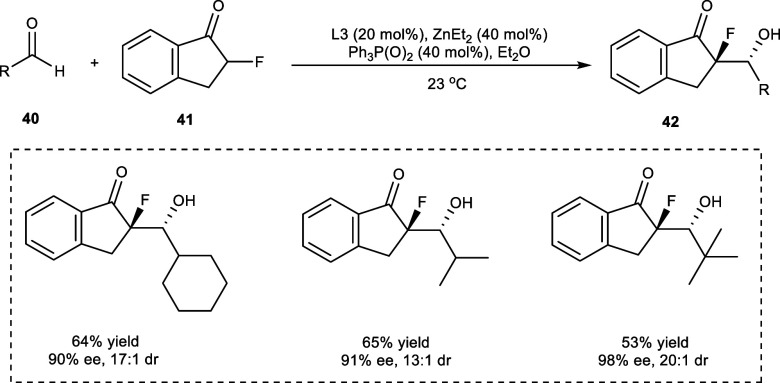
In 2016, Trost et al. synthesized tetrasubstituted C–F stereogenic centers by reacting α-branched derivatives of aldehyde (40) with α-fluoroketones (41) in the presence of a Zn-ProPhenol catalytic system (Scheme 21). The required products (42) have been obtained in high enantio- (98% ee) and diastereoselectivity (>20:1 dr). In this asymmetric reaction triphenylphosphine oxide has been added as a Lewis-basic additive playing the crucial rule in increasing the diastereoselectivity (dr) of the reaction.36
Scheme 21. Synthesis of Tetrasubstituted C–F Stereogenic Centers via Aldol Addition Reaction.
Aza-Henry Reaction
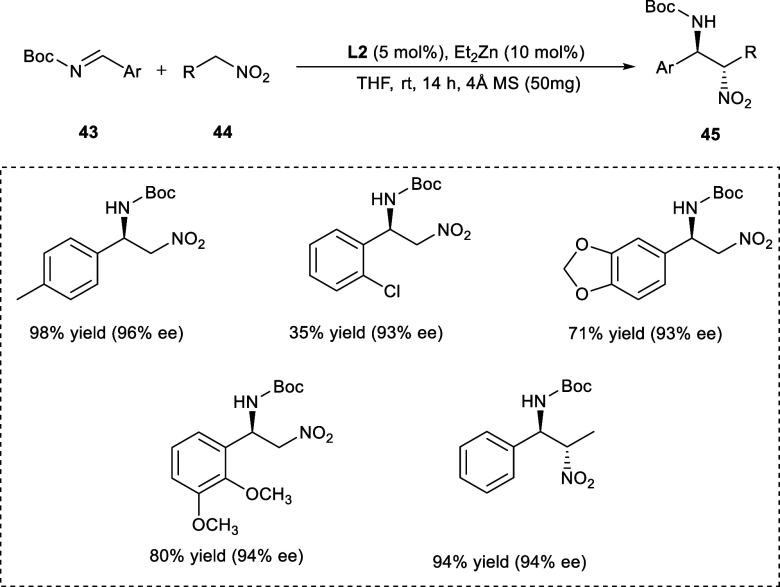
Among the most significant transformations for the synthesis of N-containing compounds is the chiral aza-nitroaldol reaction of imines and carbonyl compounds. The resulting asymmetric β-nitroamines can be readily transformed into very useful chemical compounds like vicinal diamine and α-amino acids.37 In 2019, Liu et al. synthesized various derivatives of nitroamines (45) from N-Boc imines (43) and nitroalkanes (44) via asymmetric nitro-Mannich transformation catalyzed by dinuclear zinc-AzePhenol system at low temperature (Scheme 22). The reaction affords high enantioselectivity in the presence of THF in comparison with other solvents. The reactivity was also greatly influenced by molecular sieves. The introduction of 4 Å MS resulted in high yields of the desired products. The imine derivatives having substituents on varied positions of the phenyl ring were well tolerated by the reaction conditions affording good yields with comparable enantioselectivities. The electronic properties of the substituent on the aryl ring had shown a great impact on reactivity. Electron-donating groups such as methoxy (−OCH3) and methyl (−CH3) on the aryl ring affords the products in higher yields in comparison to the electron-withdrawing group such as fluoro (−F) and chloro (−Cl). The 2,3-dimethoxyl substituted Boc-protected imine improved the enantiomeric excess of the desired product in contrast to imine having the 4-methoxy substituent.
Scheme 22. Synthesis of Nitroamines by an Asymmetric Nitro-Mannich Reaction by Employing Dinuclear Zn-Catalytic System.
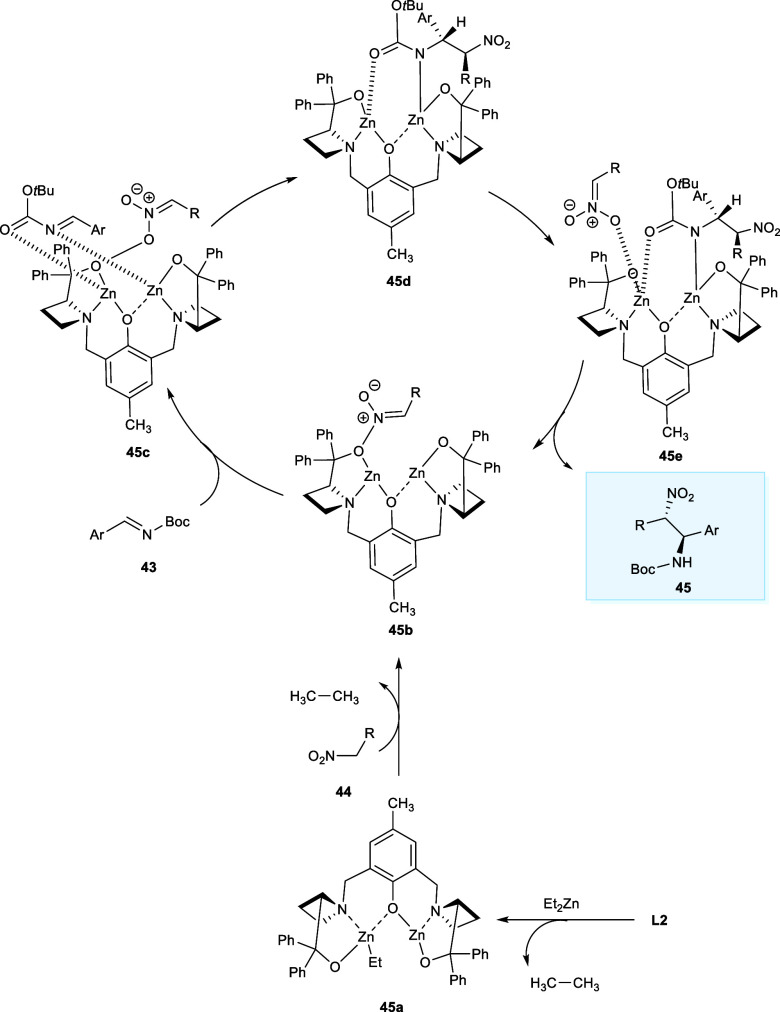
The proposed mechanism for the aza-Henry reaction involves the use of a catalyst (45a) for the deprotonation of nitromethane affording the intermediate (45b), which is zinc nitronate. The production of complex (45c) takes place by the imine coordination of Zn metal atoms to the more sterically susceptible position, which is followed by nitronate attack on the (45c) to yield intermediate complex (45d) which is responsible for the enantio- and diastereoselectivity of reaction. Then the complex (45e) has been produced by the coupling of nitromethane, which after the proton exchange with the nitroalkane regenerates the catalytic cycle, affording the desired product (45) (Scheme 23).38
Scheme 23. Cyclic Mechanism of Nitro-Mannich Reaction Catalyzed by Dinuclear Zn-AzePhenol System.
Asymmetric Amination
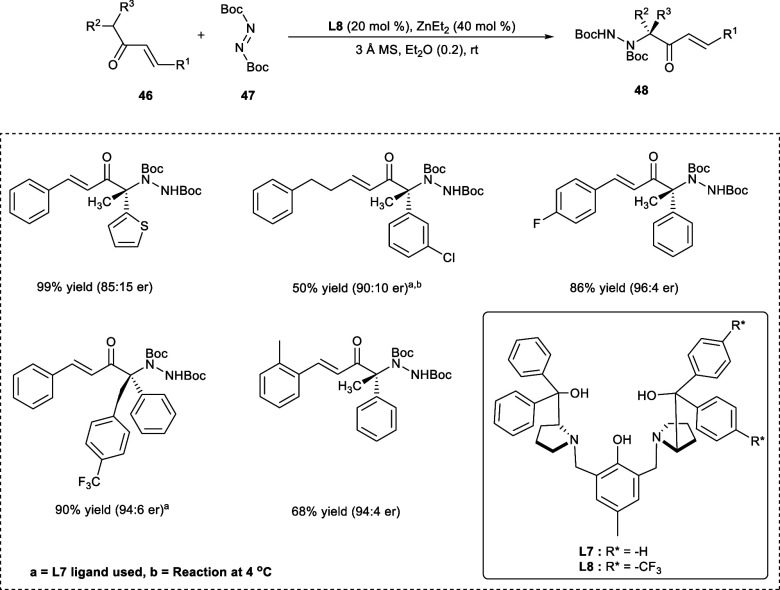
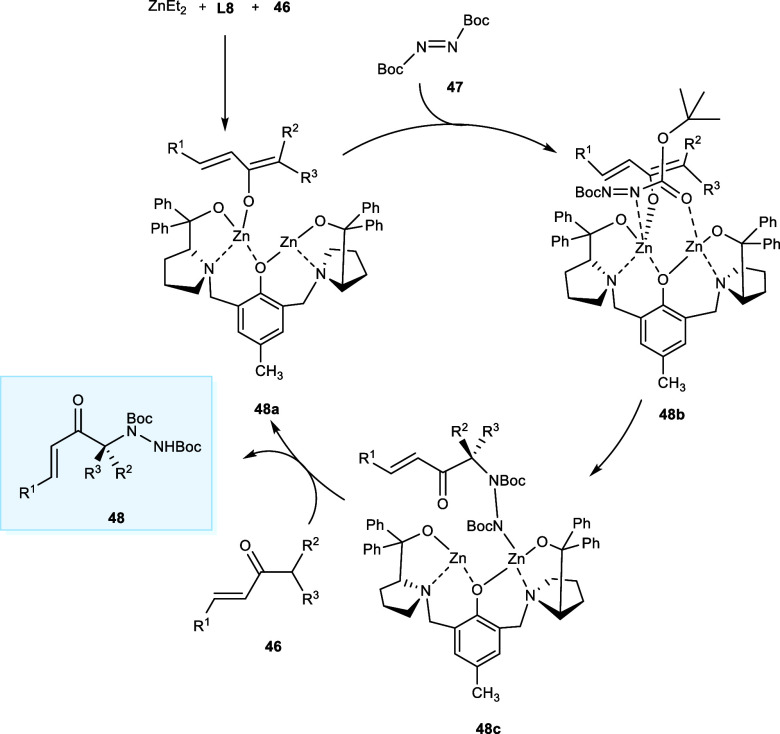
Natural products, commercial medications, and biologically active molecules all contain nitrogen-containing molecules. Consequently, in synthetic chemistry, a lot of effort has been put into establishing methods for chemically incorporating nitrogen atoms into organic molecules.7,39 In 2019, chiral α-tertiary amine related compounds (48) were synthesized via electrophilic amination of aliphatic α-branched ketone derivatives (46) using di-tert-butyl azodicarboxylate (47) in the presence of Zn-ProPhenol complex of ligand (S,S)-L8 (Scheme 24). The nitrogen of the di-tert-butyl azodicarboxylate serves as the electrophilic site where the nucleophilic attack of α-branched ketone derivatives takes place. The ligand (S,S)-L8 having electron-withdrawing groups has shown the highest selectivity rate with excellent enantiomeric ratio (er) of 96:4 and yields of desired products. The reaction is well tolerated upon the introduction of either electron-rich or electron-poor heteroaromatic or aromatic rings at the R1-position. The electron-withdrawing substituents, i.e., X = −F, −Cl, at the p-position and o-position of the R1 substituent affords the product in high yield (89–92%) with selectivity ranging from 96:4 to 97:3 er. In the case of electron-donating substituent (e.g., -OMe) at the meta-position the yields were 75% with an enantiomeric ratio (er) of 93:7. However, the yields were decreased upon the introduction of an electron-donating substituent (e.g., −Me) at the ortho-position, i.e., 68% yield with comparatively higher er of 96:4.
Scheme 24. Synthesis of α-Tertiary Amines via Amination of Vinyl Ketone Derivatives.
In asymmetric amination, the formation of the first dinuclear Zn-catalyst is followed by the deprotonation of the vinyl ketone to afford the complex (48a). Then, complex (48b) is generated after the electrophilic N atoms are coordinated with the two Zn atoms at the binding sites. The dinuclear structure of the Zn-catalyst is responsible for this distinctive chelation by using an electrophilic bidentate ligand, which corresponds to the reactivity and selectivity. Now, among (E)-enolate and (Z)-enolate, the reaction would take place via (E)-enolate reducing 1,3-diaxial repulsion among the sterically crowded Boc groups of complexes (48b) and enolate according to the Curtin–Hammett control. The resulting base, i.e., hydrazine complex (48c), will deprotonate another molecule of ketones (46), affording the desired product (48), and the catalyzed cycle is regenerated (Scheme 25).40
Scheme 25. Synthesis of α-Tertiary Amines via Amination of Vinyl Ketone Derivatives.
One-Pot Synthesis
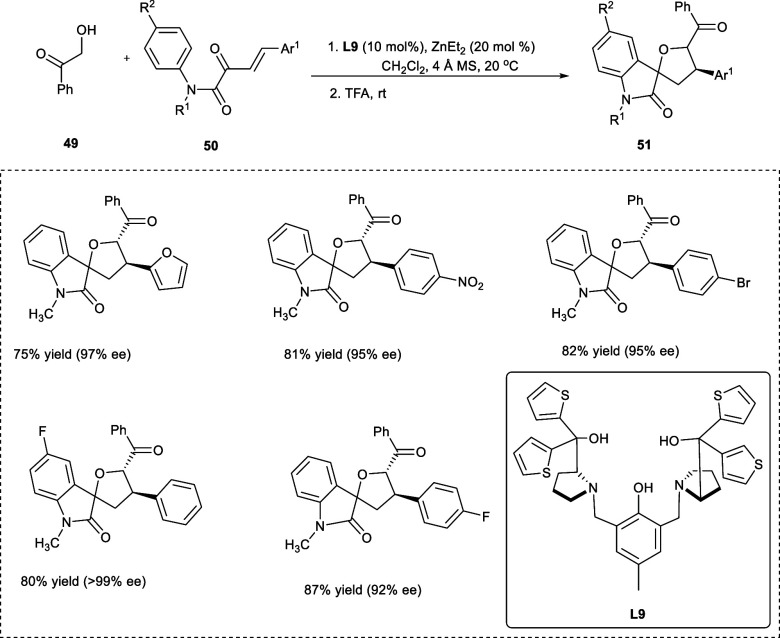
The spirooxindole, which is found in many naturally occurring and man-made compounds, has drawn the attention of researchers because of its vast applicability in both the chemical and pharmaceutical industries.41,42 Among the spirooxindoles, asymmetric tetrahydrofuran spirooxindole compounds have been found to have exemplary biological properties, such as growth inhibitory and anticancer activities.43,44 The dinuclear zinc catalyst based on ZnEt2 and an asymmetric multidentate semiazacrown ether ligand (S,S)-L9 has led to many enantioselective catalytic reactions. In 2019, Guo et al. carried out the one-pot transformation to obtain tetrahydrofuran spirooxindole-related compounds (51) from a variety of α-hydroxy phenyl ketones (49) and β,γ-unsaturated α-ketoamide derivatives (50). The reaction has been catalyzed by a dinuclear Zn-catalyst and trifluoroacetic acid (CH3CO2H) (Scheme 26). The one-pot synthesis involves asymmetric hemiketalization/Michael addition followed by Fridel Crafts reaction. A range of structurally distinct tetrahydrofuran spirooxindole derivatives has been obtained in good yields (75% to 87%) with enantioselectivity high as 99% and diastereoselectivity ratio of 3:1–13:1. The N-aromatic ring having electron-donating substituents (e.g., −Me, −OMe) or electron-withdrawing substituents (e.g., halogens) at p-position undergo reaction more effectively giving 80–85% yields of the desired products with dr ration 8:1–13:1 and ee up to >99%).
Scheme 26. Synthesis of Tetrahydrofuran Spiro Oxindoles via One-Pot Reaction Using Dinuclear Zn-Catalyst and TFA.
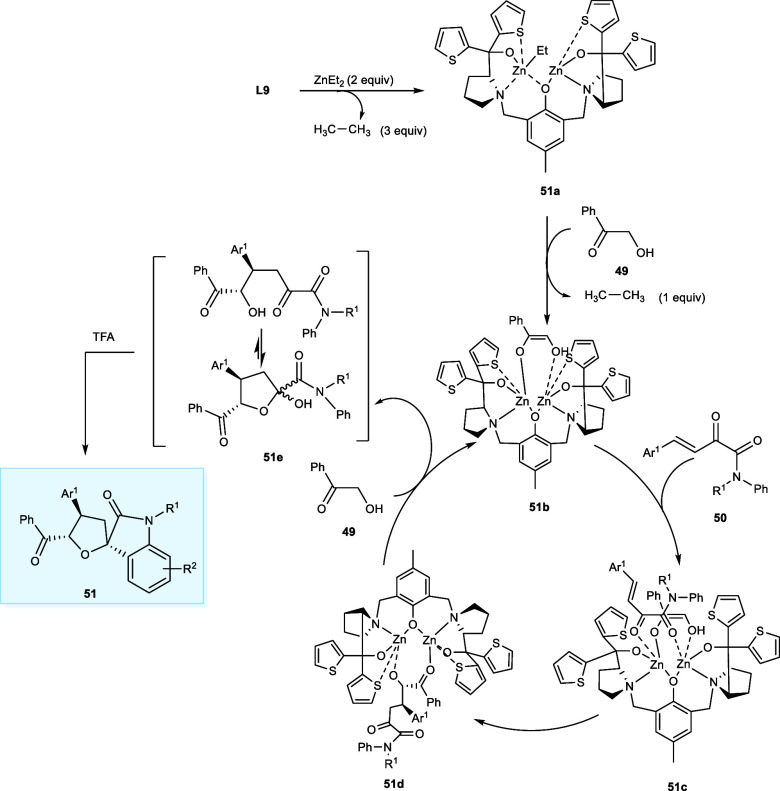
The proposed reaction cycle for the synthesis of spiro oxindole derivatives commences with the generation of an in situ dinuclear zinc complex (51a) by treating ZnEt2 with a ligand (L9). Then, the dinuclear zinc complex (51a) works as a catalyst and deprotonates the α-hydroxy acetophenone (49), yielding the intermediate (51b) with the elimination of one ethane molecule. Subsequently, the β,γ-unsaturated α-ketoamide derivative (50) undergoes coordination with the least hindered site of both the Zn metal atoms affording the complexes (51c), which further undergoes the Michael 1,4-addition to yield the complex (51d) with the examined stereochemical configuration. Then, complex (51d) upon the H+ transfer with another molecule of α-hydroxy acetophenone (49) affords the intermediate (51e), and the catalytic cycle continues. The intermediate (51e) has been the combination of cyclic structure (hemiketalization/Michael adduct) and chain structure, which is the Michael addition product. Finally, the intermediate (51e) has been treated with TFA to yield desired product by intramolecular Friedel–Crafts alkylation (Scheme 27).45
Scheme 27. Mechanistic Cycle Synthesizing the Tetrahydrofuran Spiro Oxindole Derivatives Using Dinuclear Zn-Complex and TFA.
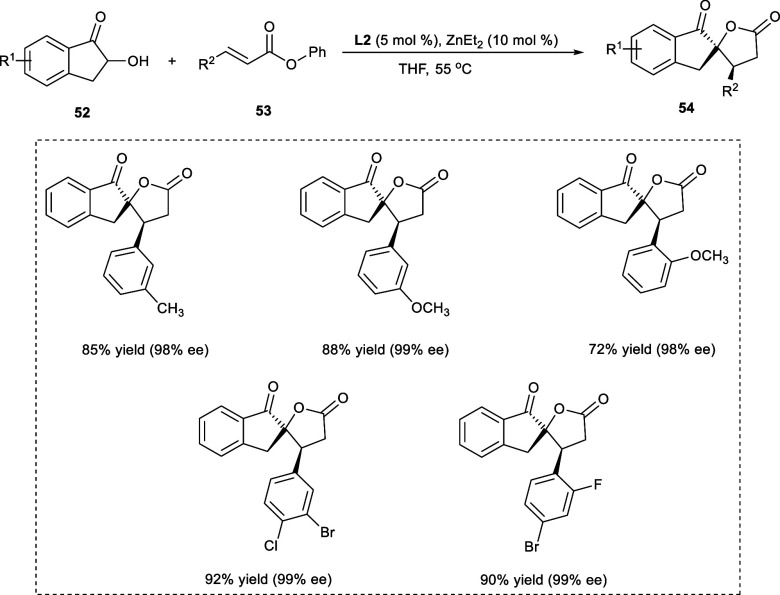
γ-Butyrolactones have been consistently seen as synthetic targets due to their widespread application in biologically active naturally occurring compounds.46 Many pharmaceutically important drugs have chiral spirocyclic butyrolactones as basic structural components; e.g., a typical drug spironolactone for treating heart failure is a synthetic steroid, and Drospirenone, also called Yasmin, is a powerful contraceptive.47 In 2019, spiro[1-indanone-5,2′-γ-butyrolactones] (53) were synthesized from α-hydroxy-1-indanones (52) and α,β-unsaturated ester derivatives (54) via a one-pot reaction catalyzed by dinuclear Zn-catalyst (Scheme 28). The one-pot reaction involves Michael 1,4-addition followed by transesterification reaction in CH2Cl2, affording the desired product with a dr ratio >20:1 and 92% ee. Both the electron-deficient and electron-rich substitutions at the 5-position of the aromatic ring of the α-hydroxy-1-indanone (52) were very well tolerated and had an insignificant impact on the yields, enantioselectivities (ee), or diastereoselectivities (dr) of the desired products.48
Scheme 28. Synthesis of γ-Butyrolactones via Zn-Catalyzed Michael 1,4-Addition Reaction.
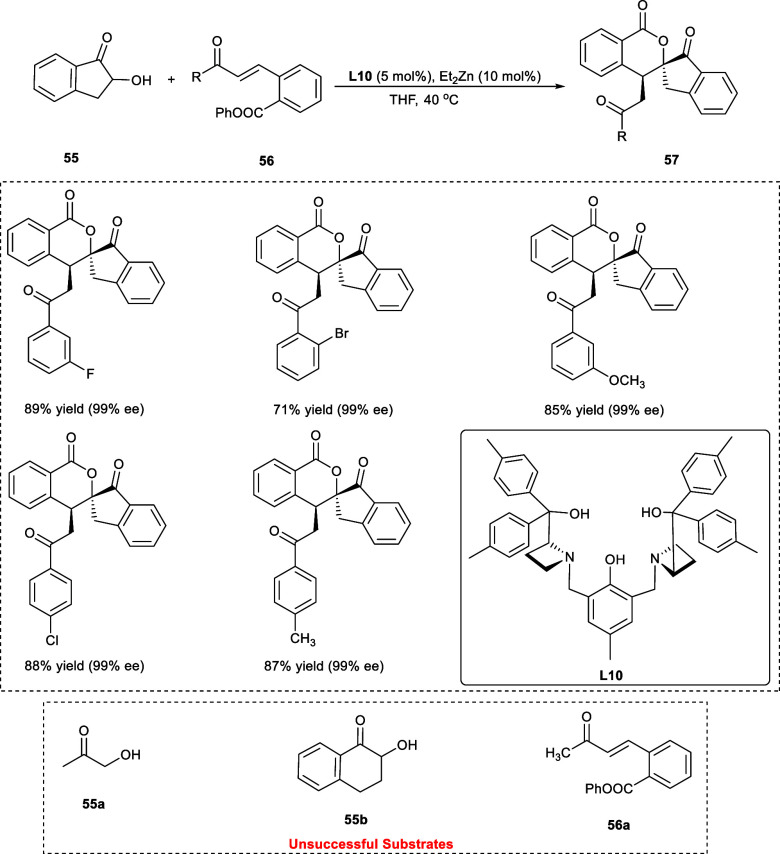
The isochromanone framework is found in a wide variety of natural products as well as pharmaceutical compounds. The isochromanone analogues, particularly chiral isochromanone molecules, exhibit a diverse range of significant bioactivities.49 An effective technique for the enantioselective and diastereoselective synthesis of spiro[indanone-2,3′-isochromane-1-one] derivatives (57) via transesterification/cascade Michael reaction of o-ester chalcones (56) with α-hydroxy indanones (55) has been reported by Yang et al. (Scheme 29). The ligand (L10) has been found most effective, yielding the targeted compounds in 92% yield exhibiting remarkable enantioselectivity and diastereoselective, i.e., >99% ee and >99:1 dr, respectively. The position and electronic nature of the substituents incorporated on the phenyl rings of α-hydroxy indanone derivatives have little impact on the stereoselectivities. Both the disubstituted and trisubstituted aromatic rings were equally tolerated under the reaction conditions. Moreover, the o-ester chalcones having a 2-naphthyl or 2-thienyl group have been shown to be appropriate substrates in this tandem approach, giving the intended products in good yield with 99% ee. Notably, nearly a single diastereoisomer has been obtained as the end product in almost all the reactions. The reaction was a failure with α-hydroxy ketone (55a) and α-hydroxy indanones (55b). Additionally, o-ester chalcones (56a) did not react, as aliphatic enones have lower reactivities compared to aromatic enones.
Scheme 29. Synthesis of Spiro[indanone-2,3′-isochromane-1-one] Derivatives via Transesterification/Cascade Michael Reaction.
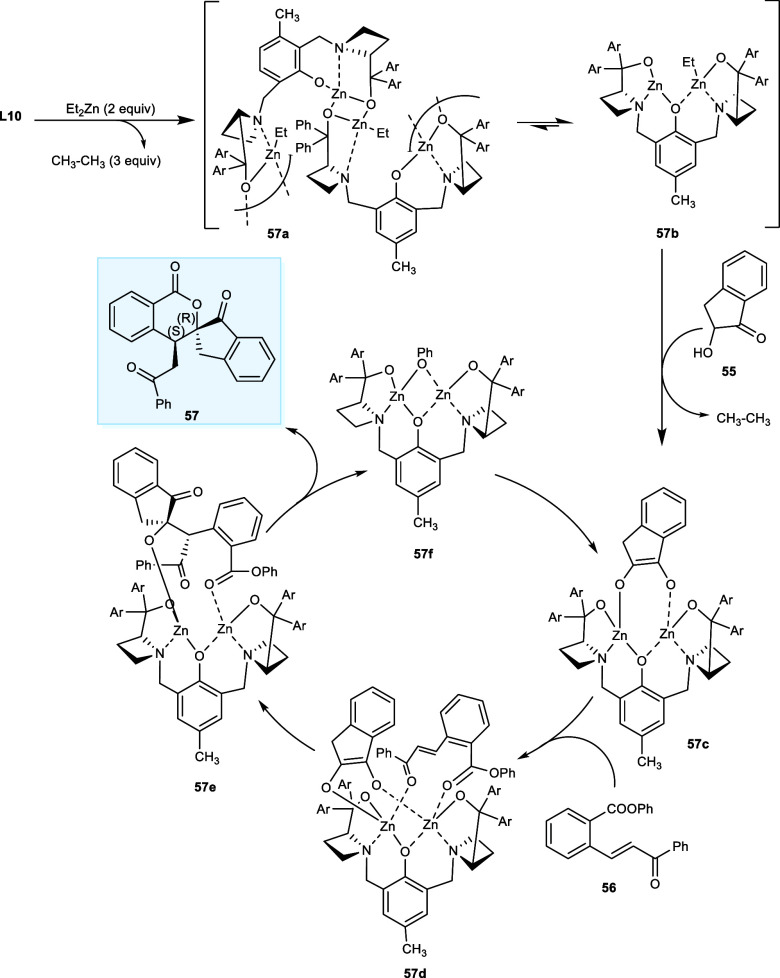
The proposed mechanism for isochromanone synthesis takes place with the formation of two dinuclear zinc complexes (57a) and (57b) by treating ZnEt2 with ligand L10. In the next step, deprotonation of α-hydroxy indanone (55) gives the catalytically active complex (57c). Now, both Zn atoms have been coupled with ortho-ester chalcones (56) from the sterically accessible side to generate the complex (57d). The formation of a carbon–carbon single bond by Michael addition generates the complex (57e). After that, complex (57e) proceeded through intramolecular transesterification, generating product (57) and zinc phenoxide species (57f). The catalytic cycle has been restarted with another deprotonation of α-hydroxy indanone (Scheme 30).50
Scheme 30. Catalytic Cycle for the Transesterification/Cascade Michael Reaction.
Endless efforts are being contributed to the developing areas of metal catalysis and organo-catalysis. Catalytic unsymmetrical multicomponent response (CAMCR) is among the foremost capable strategies for quickly increasing the stereochemical complexity. Formation of multiple bonds and numerous stereocenters have been accomplished at the same time by engineering a single-step reaction utilizing a domino response with at least three reacting components using a catalytical number of stereogenic catalysts.51
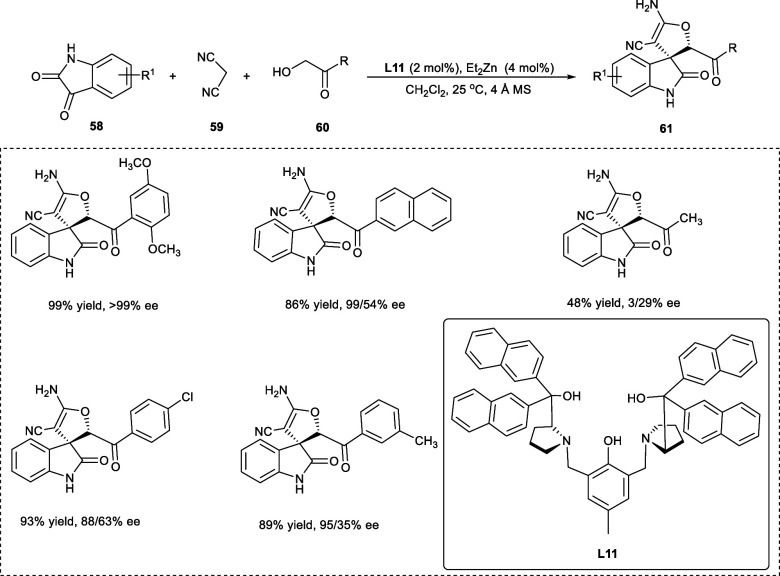
In 2019, Miao et al. utilized three components; isatin (58), malononitrile (59), and α-hydroxy acetophenone (60) in a domino reaction to synthesize chiral 3,3′-dihydrofuran spirooxindole derivatives (61) in excellent yield with enantioselectivities (ee) up to 99% (Scheme 31). The domino reaction begins with Knoevenagel condensation/Michael addition, followed by cyclization in CH2Cl2. The stereoselectivity of the desired product decreases upon utilizing isatin-bearing N–Me or N–Bn protecting groups. The reaction seems to be well tolerated in the case of several electron-rich as well electron-deficient groups on α-hydroxy acetophenone with excellent ee values. However, in the case of isatins, the presence of an electron-withdrawing group decreases the enantioselectivities.
Scheme 31. Synthesis of 3,3′-Dihydrofuran Spirooxindole Derivatives by Domino Reaction.
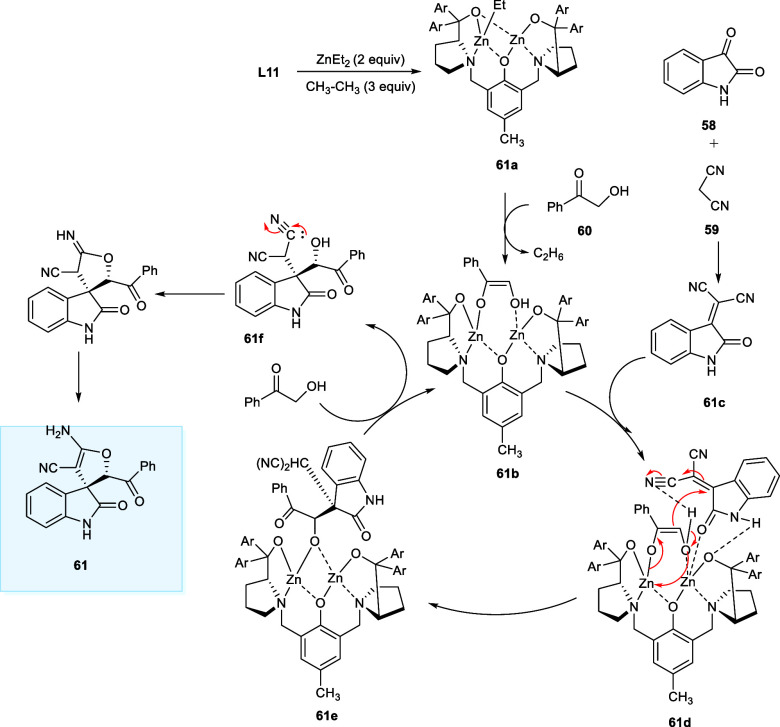
The Zn-catalyzed cycle for spirooxindole synthesis commences with the formation of ethylzinc complex (61a) which deprotonated the α-hydroxy acetophenone (60) to generate bidentate bridging enolate (61b). Next, the Knoevenagel condensation product (61c) from (58) and (59) afford the intermediate (61d) upon coordinating with the sterically less hindered Zn atom via the indicated orientation. In this well-regulated chiral environment, intermediate (61e) has been generated through the parallel generation of new carbon–carbon single bonds and asymmetrical centers, via a Michael 1,4-addition reaction and tautomerization. Lastly, the catalytic cycle undergoes completion upon the H+ transfer from α-hydroxy acetophenone (60) nucleophile affording an unstable product (61f). The unstable (61f) undergoes Pinner transformation/isomerization to afford the stable desired product (Scheme 32).52
Scheme 32. Catalytic Cycle of Three-Component Domino Reaction.
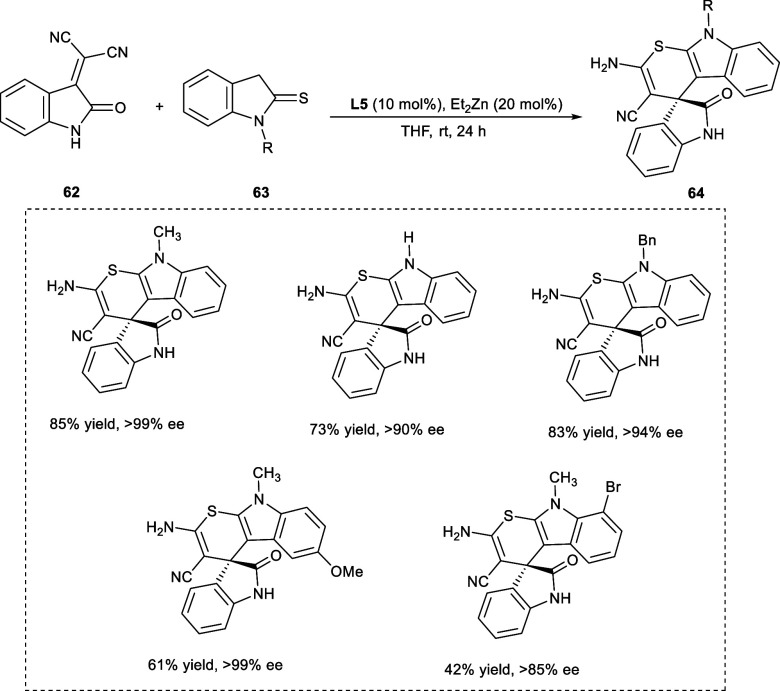
In 2022, Liu et al. employed a binuclear Zn-ProPhenol catalytic system to synthesize biologically active chiral spiro[indoline-3,40-thiopyrano[2,3-b]indole] compounds (64) from isatylidene malononitriles (62) and indoline-2-thiones (63) via asymmetric cascade [3 + 3] cyclization reaction (Scheme 33). The indoline-2-thiones (62) having either electron-rich or electron-deficient groups at the 5-position have been tolerated well, giving the products in moderate to good yields with remarkable ee values (87–99%). Additionally, electron-withdrawing substitutions at the 6-position (−Cl, −Br) and 7-position (−F, −Br) were also tolerated well. Notably, the incorporation of an electron-withdrawing substituent (−Br) at either the 5- or 7-position has shown a negative impact on the product yield as well as the enantioselectivity (ee) of the reaction.53
Scheme 33. Synthesis of Spiro[indoline-3,40-thiopyrano[2,3-b]indole] Derivatives by Catalytic Asymmetric Cascade [3 + 3] Cyclization Reaction.
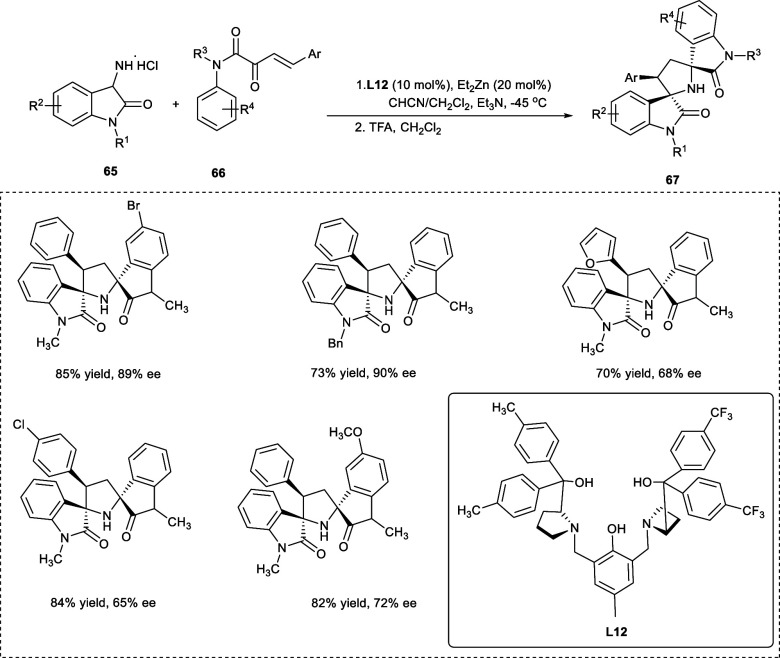
The 2,3-pyrrolidinyl spirooxindole skeletons are prominent structural motifs that are present in a variety of natural as well as synthetic organic molecules.100−300 These optically pure 2,3-pyrrolidinyl spirooxindoles are found to be responsible for remarkable biological and pharmacological properties.400,500 The 2,5-pyrrolidinyl dispirooxindole derivatives (67) have been synthesized from 3-amino oxindole hydrochlorides (65) and β,γ-unsaturated α-keto amide (66) upon employing asymmetric semiaza crown ether ligand (S,S-L12) in a tandem reaction (Scheme 34).
Scheme 34. Synthesis of 2,5-Pyrrolidinyl Di-spirooxindoles Using Semi-aza Crown Ether Ligand via Tandem Reaction.
The reaction involves Michael/cyclic keto-imine transformation followed by Friedel–Crafts reaction affording pyrrolidinyl dispirooxindole derivatives having two nonadjacent spiro-quaternary stereogenic centers at C-2 and C-5 of the pyrrolidine aromatic ring. Acetonitrile has been the best-suited solvent for the reaction, giving enantioselectivity up to 76% in good yields of the desired products. The ee values have also been improved upon lowering of temperature up to −45 °C. The starting substrates having electron-rich groups (−CH3, −OCH3) at the p-position of the aryl ring (Ar) have given better enantioselectivity than the electron-deficient groups (−F, −Cl, −Br, −NO2). However, both electron-rich and electron-deficient substitutes were tolerated well at R4 and the p-position of the phenyl ring (at the N-atom), furnishing good yields of the products with moderate to good ee.54
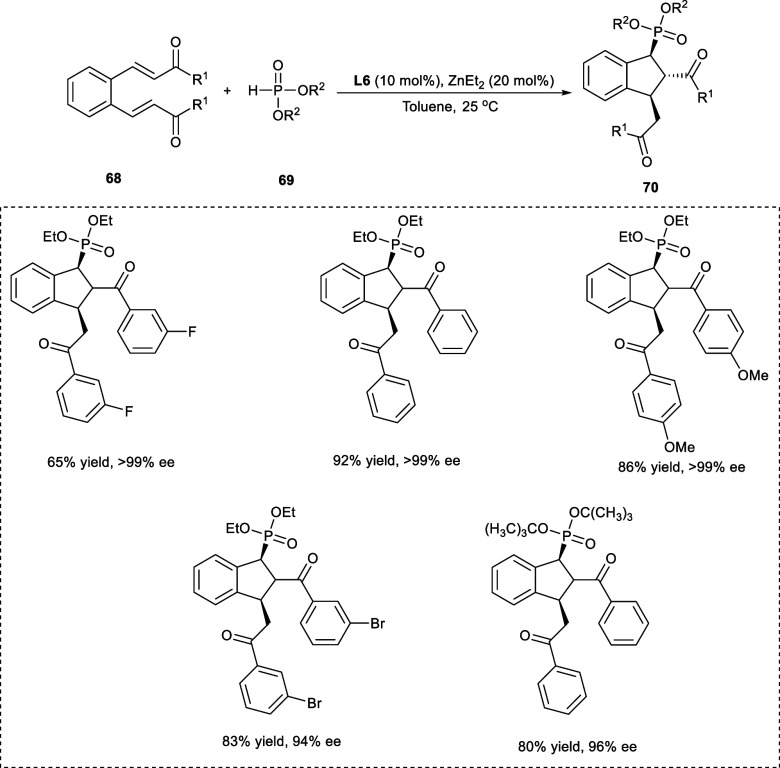
Indane has been identified in multiple natural and man-made compounds as the core skeleton. Indane derivatives, particularly compounds with chiral Indane, have drawn the interest of chemists across a wide variety of fields because of their unusual biological characteristics.55 In 2019, Tao et al. synthesized 1,2,3-trisubstituted indane derivatives (70) having phosphoryl group from o-dienones (68) and diethyl phosphate (69) via Michael tandem/phospha-Michael addition reaction by employing Zn-catalyst (Scheme 35). The targeted products were afforded with high stereoselectivities, i.e., dr up to >99:1 and ee >99%. The electronic properties of the substituents incorporated upon the phenyl ring (R1) have shown negligible impact on stereoselectivity. The substrate having either an electron-rich group (−OMe) or electron-withdrawing group (−F) at the 3-position on the aromatic ring exhibits reduced yields, i.e., below 80%. However, the substrates having an electron-withdrawing substituent (−Br) at either the m-position or p-position yield the products with slightly lower ee values i.e., 94%–95%.
Scheme 35. Synthesis of 1,2,3-Tri-substituted Indanes via Michael Tandem/Phospha-Michael Reaction.
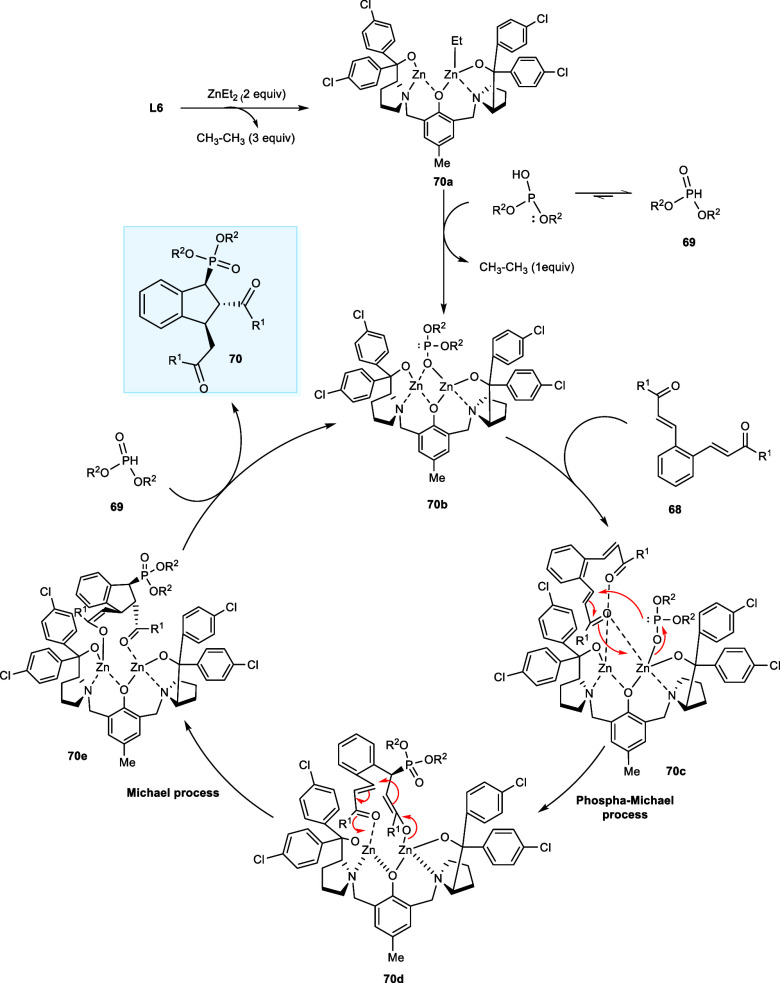
The reaction commences with the formation of dinuclear zinc complex (70a) by treating ligand L6 with ZnEt2 followed by the deprotonation of the diethyl phosphonate (69) by (70a) generating intermediate (70b) upon the removal of one ethane molecule. In the next step, complex (70c) is afforded upon the coordination of o-dienone (68) to the zinc metal from the least hindered site. After that, the phospha-Michael reaction gives the intermediate (70d), which promptly generates the intermediate (70e) via a Michael addition reaction. Lastly, a proton transfer between (70e) and another diethyl phosphonate (69) molecule affords the required product (70) and intermediate (70b), and the catalytic cycle restarts (Scheme 36).56
Scheme 36. Mechanism for the Michael Tandem/Phospha-Michael Reaction.
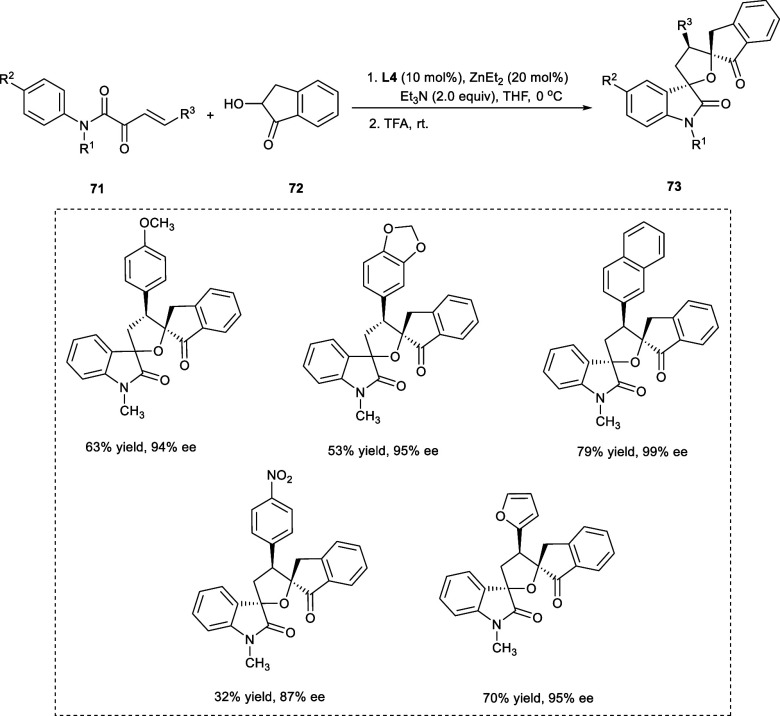
Spirooxindoles are found in a variety of organic bioactive compounds. The chiral spirooxindoles having a tetrahydrofuran backbone have been found to have anticancer potential.42,43,57,58 A versatile and effective technique facilitated by a dinuclear Zn-AzePhenol for the formation of oxindoles bispiro[3,2′-tetrahydrofuran-5′,2″-indanone] (73) via a one-pot reaction from 2-hydroxy-1-indanone (72) and β,γ-unsaturated α-ketoamide (71) has been reported by Liu et al. (Scheme 37). The one-pot reaction involves Michael/hemiketalization followed by an intramolecular Friedel–Crafts reaction. The results evidenced that enantiomeric selectivity (ee), and yields were significantly influenced by the temperature. A decrease in the temperature to 0 °C led to a higher enantioselectivity value of 95%. However, upon further dropping the temperature to either −10 or −20 °C, both the stereoselectivities and yields have been diminished. Furthermore, using triethylamine (NEt3) as an additive has shown a significant improvement in enantioselectivity (ee) and diastereoselectivity (dr), i.e., 98% ee and dr ratio of 17:1, respectively. The reaction proceeds smoothly upon the incorporation of either electron-donating (−CH3, −OCH3) or electron-withdrawing (−F, -C, -Br) substituents upon the aryl nucleus at the R3-position. However, upon incorporating a strong electron-deficient group (−NO2), the reactivity has been reduced considerably, diminishing the yield (32%) as well as the enantioselectivity (87%) of the desired products.57
Scheme 37. Synthesis of Oxindoles Bispiro[3,2′-tetrahydrofuran-5′,2″-indanone] via Catalytic One-Pot Reaction.
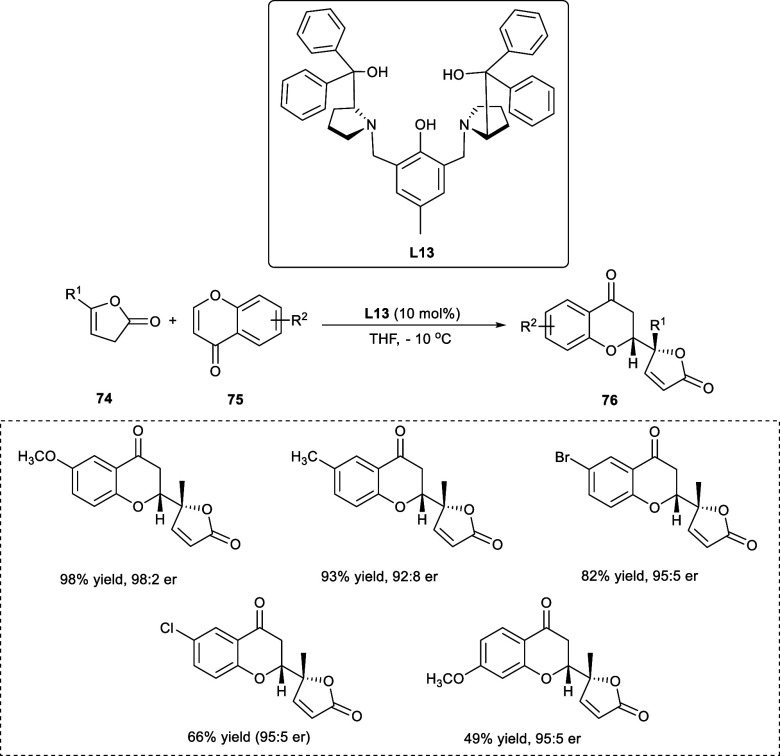
The chromanone lactones are naturally occurring compounds composed of 4-chromanones with a five-membered lactone ring at the 2-position.59 The chromanone lactone family contains various biologically active compounds, for example, Blennolides E and D, exhibiting antialgal and antifungal properties.60 Although Zn-ProPhenol as a catalyst for 1,2-additions of imines to aldehydes is widely utilized, 1,4-addition reactions catalyzed by Zn-ProPhenol are relatively uncommon. In 2019, Trost et al. utilized the 1,4-asymmetric addition reaction of butenolides (74) to chromones (75) affording the vinylogous derivatives of the addition product (76) (Scheme 38).
Scheme 38. Asymmetric 1,4-Addition of Butanolide Derivatives to the Chromone Nucleus.
The chromones having a halogen (−Cl, −Br) at the 6-position have shown excellent ee and dr values. However, chromones having electron-rich substituents at the 6-position have been the best electrophiles for the transformation. The reactivity of the reaction has been reduced upon the incorporation of the electron-donating substituent at the 7-position, although enantioselectivity and diastereoselectivity were not affected.59
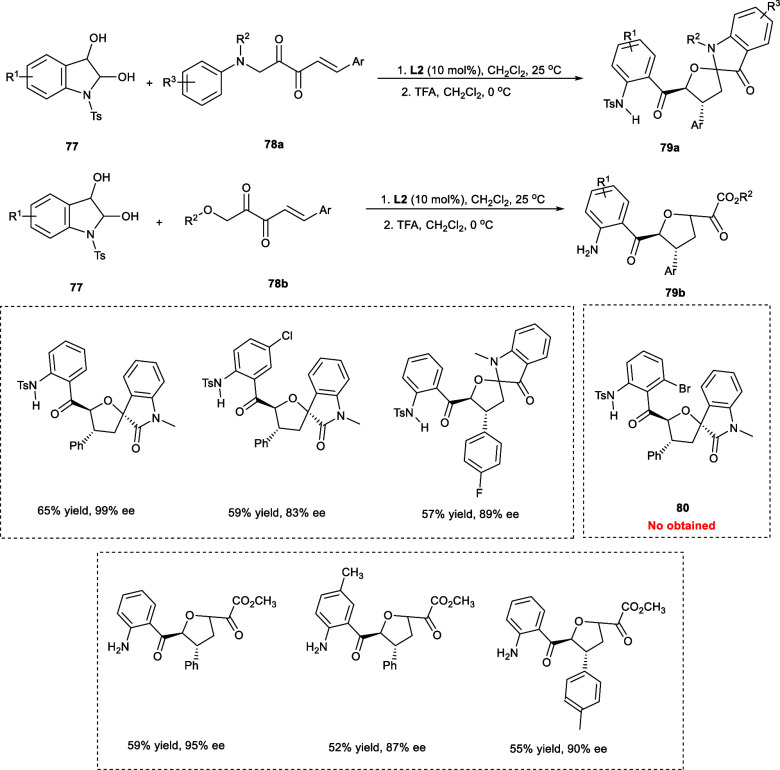
Xiang et al. reported the synthesis of numerous structurally diverse tetrahydrofuran spirooxindoles (78a) and dihydrofurans (78b) from β,γ-unsaturated-α-keto derivatives (79a–b) and 1-tosylindoline-2,3-diols (77) via an asymmetrical tandem reaction catalyzed by dinuclear Zn-catalyst. The hemiacetals as α-carbon nucleophiles have been reported to exhibit umpolung activity through chiral dinuclear zinc catalysis (Scheme 39). The 1-tosylindoline-2,3-diol derivatives having either electron-rich or electron-withdrawing substitutions at the 5-positions performed well during the reaction yielding the desired products from 46% to 59% with high stereoselectivities, i.e., d.r. of 20/1 and ee from 83% to 92%. However, upon the incorporation of −Br at the 4-position of the aromatic ring (80), no reaction has been observed due to steric crowding. The presence of both electron-rich (−OMe, −Me) and electron-withdrawing (−F, −Cl, −Br) substitution were also effectively tolerated at p-position (R3) in the N-aromatic ring backbone affording the intended products in fair yields with 90–96% ee. The reaction also proceeded smoothly in the presence of a variety of substituents on β,γ-unsaturated-α-keto (Ar), including heterocyclic substituents.
Scheme 39. Synthesis Involving Hemiacetals As α-Carbon Nucleophiles.
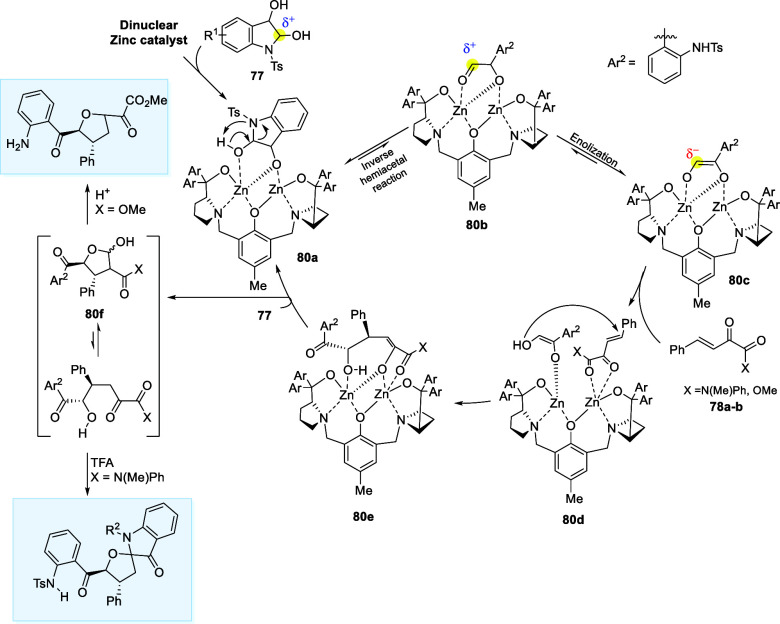
The reaction commences with the formation of intermediate (80a) via deprotonation of (77). Subsequently, the intermediate (80a) is converted to intermediate (80b) via slow reverse hemiketal reaction, followed by immediate enolization of (80b), affording the enediol intermediate (80c) which serves as the α-carbon nucleophile, demonstrating the umpolung behavior of hemiacetal. The electrophilic β,γ-unsaturated α-keto derivatives (78a–b) has been activated through Zn–O coordination, leading to the formation of the complex (80d) from the sterically most accessible side. Subsequently, (80d) undergoes a Michael 1,4-addition, forming intermediate (80e) with the stereochemical configuration. Lastly, proton transfer with the second molecule of (77) releases the product of the Michael reaction (80f), and the cycle is initiated once again. Finally, treatment of (80f) with acid affords desired products (79a–b) (Scheme 40).61
Scheme 40. Catalytic Cycle Involving Umpolung of Hemiacetal.
Conclusion
In conclusion, the bimetallic ProPhenol Zn complexes exhibit a wide range of capabilities as catalysts, enabling a diverse set of asymmetric transformations for the production of valuable chemical components. The Brønsted basic center of these catalysts facilitates direct enolization/deprotonation of pronucleophilic moieties, allowing the asymmetric addition reactions without the need for prior activation. For instance, various derivatives of ketone can be directly utilized as nucleophiles in Mannich and aldol reactions, eliminating the requirement for the preformation of corresponding silyl enol ethers or enolates. Furthermore, the Lewis acidic center enhances the stereocontrol upon coordination with the electrophilic moieties. To date, many asymmetric transformations have been devised using metal (M)-ProPhenol catalysts, encompassing direct aldol, Aza-Henry, Mannich reactions, conjugate additions, alkynylations, and the one-pot process. Also, a significant number of documented procedures have been employed in the total synthesis of natural substances and various biologically active compounds. Despite extensive investigations into the chemistry of ProPhenol ligands in the last two decades, there are still areas that offer room for development. For example, the scope of electrophilic substances remains primarily limited to simple aldimines and aldehydes. One approach to overcome the limited reactivity of bulkier electrophilic substances is to modify the chiral environment of the catalyst by adjusting the ligand substitution. Despite the introduction of several new generations of ProPhenol ligands, the highly modular nature of the ligand framework should enable the exploration of more innovative designs involving zinc as well as other metals. Exploring this avenue promises to be a captivating area of research, leading to new asymmetric processes.
Acknowledgments
The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through research group program under grant number RGP-2/569/44.
The authors declare no competing financial interest.
References
- Rudnick R. L.; Fountain D. M. Nature and composition of the continental crust: a lower crustal perspective. Reviews of geophysics 1995, 33 (3), 267–309. 10.1029/95RG01302. [DOI] [Google Scholar]; Rosman K. A survey of the isotopic and elemental abundance of zinc. Geochim. Cosmochim. Acta 1972, 36 (7), 801–819. 10.1016/0016-7037(72)90089-0. [DOI] [Google Scholar]; Li W.; Gou W.; Li W.; Zhang T.; Yu B.; Liu Q.; Shi J. Environmental applications of metal stable isotopes: Silver, mercury and zinc. Environ. Pollut. 2019, 252, 1344–1356. 10.1016/j.envpol.2019.06.037. [DOI] [PubMed] [Google Scholar]
- Qi X.; Li Y.; Zhang G.; Li Y.; Lei A.; Liu C.; Lan Y. Dinuclear versus mononuclear pathways in zinc mediated nucleophilic addition: a combined experimental and DFT study. Dalton transactions 2015, 44 (24), 11165–11171. 10.1039/C5DT01366F. [DOI] [PubMed] [Google Scholar]
- Hamer W. J.Standard cells: Their construction, maintenance, and characteristics; US Government Printing Office, 1965. [Google Scholar]; Hall G. E.Purification and Biochemical Characterization of Carbonic Anhydrase from Erythrocytes of Salmo Gairdneri; The Pennsylvania State University, 1982. [Google Scholar]
- Busacca C. A.; Fandrick D. R.; Song J. J.; Senanayake C. H. The growing impact of catalysis in the pharmaceutical industry. Advanced Synthesis & Catalysis 2011, 353 (11–12), 1825–1864. 10.1002/adsc.201100488. [DOI] [Google Scholar]; Wender P. A.; Verma V. A.; Paxton T. J.; Pillow T. H. Function-Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res. 2008, 41, 40–49. 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- Trost B. M. The atom economy—a search for synthetic efficiency. Science 1991, 254 (5037), 1471–1477. 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- Tsuji J.Modern rhodium-catalyzed organic reactions; John Wiley & Sons, 2005. [Google Scholar]; Murahashi S.-I.; Takaya H.; Naota T. Ruthenium catalysis in organic synthesis. Pure and applied chemistry 2002, 74 (1), 19–24. 10.1351/pac200274010019. [DOI] [Google Scholar]; Liu Z.; Sadler P. J. Organoiridium complexes: anticancer agents and catalysts. Acc. Chem. Res. 2014, 47 (4), 1174–1185. 10.1021/ar400266c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayesha; Bilal M.; Rasool N.; Khan S. G.; Rashid U.; Altaf H.; Ali I. Synthesis of indoles via intermolecular and intramolecular cyclization by using Palladium-based catalysts. Catalysts 2021, 11 (9), 1018. 10.3390/catal11091018. [DOI] [Google Scholar]
- Wu X. F.; Neumann H. Zinc-Catalyzed Organic Synthesis: C—C, C—N, C—O Bond Formation Reactions. Advanced Synthesis & Catalysis 2012, 354 (17), 3141–3160. 10.1002/adsc.201200547. [DOI] [Google Scholar]; Ahmad H.; Bilal M.; Maqbool T.; Rasool N.; Adnan Ali Shah S.; Amiruddin Zakaria Z. Recent advances on Copper-catalyzed Asymmetric Synthesis and Their Potential Biological Applications. Journal of Saudi Chemical Society 2023, 27, 101658. 10.1016/j.jscs.2023.101658. [DOI] [Google Scholar]; Bolm C.; Legros J.; Le Paih J.; Zani L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104 (12), 6217–6254. 10.1021/cr040664h. [DOI] [PubMed] [Google Scholar]; Alexakis A.; Krause N.; Woodward S.. Copper-catalyzed asymmetric synthesis; John Wiley & Sons, 2013. [Google Scholar]
- Trost B. M.; Bartlett M. J. ProPhenol-catalyzed asymmetric additions by spontaneously assembled dinuclear main group metal complexes. Acc. Chem. Res. 2015, 48 (3), 688–701. 10.1021/ar500374r. [DOI] [PMC free article] [PubMed] [Google Scholar]; Trost B. M.; Ito H. A direct catalytic enantioselective aldol reaction via a novel catalyst design. J. Am. Chem. Soc. 2000, 122 (48), 12003–12004. 10.1021/ja003033n. [DOI] [Google Scholar]; Trost B. M.; Hung C. I.; Mata G. Dinuclear metal-prophenol catalysts: development and synthetic applications. Angew. Chem., Int. Ed. 2020, 59 (11), 4240–4261. 10.1002/anie.201909692. [DOI] [PubMed] [Google Scholar]
- Mahrwald R.Modern Aldol Reactions. 2004. [Google Scholar]; Geary L. M.; Hultin P. G. The state of the art in asymmetric induction: the aldol reaction as a case study. Tetrahedron: Asymmetry 2009, 20 (2), 131–173. 10.1016/j.tetasy.2008.12.030. [DOI] [Google Scholar]; Schetter B.; Mahrwald R. Modern aldol methods for the total synthesis of polyketides. Angew. Chem., Int. Ed. 2006, 45 (45), 7506–7525. 10.1002/anie.200602780. [DOI] [PubMed] [Google Scholar]; Faber K.; Turner N. J.; Fessner W.-D.. Science of Synthesis: Biocatalysis in Organic Synthesis; Georg Thieme Verlag, 2015. [Google Scholar]
- Zhang Y.-F.; Yin S.-J.; Zhao M.; Zhang J.-Q.; Li H.-Y.; Wang X.-W. Dinuclear zinc-catalyzed desymmetric intramolecular aldolization: an enantioselective construction of spiro [cyclohexanone-oxindole] derivatives. RSC Adv. 2016, 6 (36), 30683–30689. 10.1039/C6RA02296K. [DOI] [Google Scholar]
- Wu X.; Corcoran C.; Yang S.; Xiao J. A versatile iridium catalyst for aldehyde reduction in water. ChemSusChem: Chemistry & Sustainability Energy & Materials 2008, 1 (1–2), 71–74. 10.1002/cssc.200700086. [DOI] [PubMed] [Google Scholar]; West L. M.; Northcote P. T.; Battershill C. N. Peloruside A: a potent cytotoxic macrolide isolated from the new zealand marine sponge Mycale sp. Journal of organic chemistry 2000, 65 (2), 445–449. 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]; Evans D. A.; Welch D. S.; Speed A. W.; Moniz G. A.; Reichelt A.; Ho S. An aldol-based synthesis of (+)-peloruside a, a potent microtubule stabilizing agent. J. Am. Chem. Soc. 2009, 131 (11), 3840–3841. 10.1021/ja900020a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Trost B. M.; Probst G. D.; Schoop A. Ruthenium-catalyzed Alder ene type reactions. A formal synthesis of alternaric acid. J. Am. Chem. Soc. 1998, 120 (36), 9228–9236. 10.1021/ja981540n. [DOI] [Google Scholar]
- Zhang Z. F.; Yang X. C.; Lu H. J.; Wang M. C. Enantioselective Direct Synthesis of syn-and anti-α, β-Dihydroxy γ-Keto Esters Using a Dinuclear Zinc–AzePhenol Complex. Eur. J. Org. Chem. 2018, 2018 (6), 785–793. 10.1002/ejoc.201701695. [DOI] [Google Scholar]
- Corey E. J.; Guzman-Perez A. The catalytic enantioselective construction of molecules with quaternary carbon stereocenters. Angew. Chem., Int. Ed. 1998, 37 (4), 388–401. . [DOI] [PubMed] [Google Scholar]; Douglas C. J.; Overman L. E. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (15), 5363–5367. 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Das J. P.; Marek I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 2011, 47 (16), 4593–4623. 10.1039/c0cc05222a. [DOI] [PubMed] [Google Scholar]; Arend M.; Westermann B.; Risch N. Modern variants of the Mannich reaction. Angew. Chem., Int. Ed. 1998, 37 (8), 1044–1070. . [DOI] [PubMed] [Google Scholar]
- Tramontini M.; Angiolini L. Further advances in the chemistry of Mannich bases. Tetrahedron 1990, 46 (6), 1791–1837. 10.1016/S0040-4020(01)89752-0. [DOI] [Google Scholar]
- Trost B. M.; Saget T.; Hung C.-I. Direct catalytic asymmetric Mannich reactions for the construction of quaternary carbon stereocenters. J. Am. Chem. Soc. 2016, 138 (11), 3659–3662. 10.1021/jacs.6b01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow E. A.; Brinker M.; Teichmann T.; Fritz E.; Kaiser W.; Brosché M.; Kangasjärvi J.; Jiang X.; Polle A. Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiology 2005, 139 (4), 1762–1772. 10.1104/pp.105.069971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil J.; Echeverria P.-G.; Reymond S.; Phansavath P.; Ratovelomanana-Vidal V.; Guerinot A.; Cossy J. Synthetic studies toward the C14–C29 fragment of mirabalin. Org. Lett. 2016, 18 (18), 4534–4537. 10.1021/acs.orglett.6b02162. [DOI] [PubMed] [Google Scholar]; Rognan D.; Boulanger T.; Hoffmann R.; Vercauteren D. P.; Andre J. M.; Durant F.; Wermuth C. G. Structure and molecular modeling of GABAA receptor antagonists. Journal of medicinal chemistry 1992, 35 (11), 1969–1977. 10.1021/jm00089a005. [DOI] [PubMed] [Google Scholar]; Beutler J.; Karbon E.; Brubaker A.; Malik R.; Curtis D.; Enna S. Securinine alkaloids: a new class of GABA receptor antagonist. Brain Res. 1985, 330 (1), 135–140. 10.1016/0006-8993(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Gnanamani E.; Tracy J. S.; Kalnmals C. A. Zn-ProPhenol catalyzed enantio-and diastereoselective direct vinylogous Mannich reactions between α, β-and β, γ-butenolides and aldimines. J. Am. Chem. Soc. 2017, 139 (50), 18198–18201. 10.1021/jacs.7b11361. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Hung C.-I. J.; Gnanamani E. Tuning the reactivity of ketones through unsaturation: Construction of cyclic and acyclic quaternary stereocenters via Zn-ProPhenol catalyzed mannich reactions. ACS Catal. 2019, 9 (2), 1549–1557. 10.1021/acscatal.8b04685. [DOI] [Google Scholar]
- Sivappa R.; Hernandez N. M.; He Y.; Lovely C. J. Studies toward the total synthesis of axinellamine and massadine. Org. Lett. 2007, 9 (20), 3861–3864. 10.1021/ol0711568. [DOI] [PubMed] [Google Scholar]; Clarke P. A.; Cridland A. P.; Rolla G. A.; Iqbal M.; Bainbridge N. P.; Whitwood A. C.; Wilson C. Studies on the Synthesis of the ABC Rings of (±)-Hexacyclinic Acid. Journal of Organic Chemistry 2009, 74 (20), 7812–7821. 10.1021/jo901547k. [DOI] [PubMed] [Google Scholar]; Sivappa R.; Mukherjee S.; Dias H. R.; Lovely C. J. Studies toward the total synthesis of the oroidin dimers. Organic & Biomolecular Chemistry 2009, 7 (16), 3215–3218. 10.1039/b909482b. [DOI] [PubMed] [Google Scholar]; Dubey S.; Bhosle P. A. Pyridazinone: an important element of pharmacophore possessing broad spectrum of activity. Medicinal Chemistry Research 2015, 24, 3579–3598. 10.1007/s00044-015-1398-5. [DOI] [Google Scholar]; Akhtar W.; Shaquiquzzaman M.; Akhter M.; Verma G.; Khan M. F.; Alam M. M. The therapeutic journey of pyridazinone. European journal of medicinal chemistry 2016, 123, 256–281. 10.1016/j.ejmech.2016.07.061. [DOI] [PubMed] [Google Scholar]
- Geng Y. H.; Hua Y. Z.; Jia S. K.; Wang M. C. Direct Asymmetric α-Selective Mannich Reaction of β, γ-Unsaturated Ketones with Cyclic α-Imino Ester: Divergent Synthesis of Cyclocanaline and Tetrahydro Pyridazinone Derivatives. Chemistry–A European Journal 2021, 27 (16), 5130–5135. 10.1002/chem.202100284. [DOI] [PubMed] [Google Scholar]
- Singh J.; Conzentino P.; Cundy K.; Gainor J. A.; Gilliam C. L.; Gordon T. D.; Johnson J. A.; Morgan B. A.; Schneider E. D.; Wahl R. C.; et al. Relationship between structure and bioavailability in a series of hydroxamate based metalloprotease inhibitors. Bioorg. Med. Chem. Lett. 1995, 5 (4), 337–342. 10.1016/0960-894X(95)00031-N. [DOI] [Google Scholar]; Kawasaki T.; Enoki H.; Matsumura K.; Ohyama M.; Inagawa M.; Sakamoto M. First total synthesis of dragmacidin A via indolylglycines. Org. Lett. 2000, 2 (19), 3027–3029. 10.1021/ol006394g. [DOI] [PubMed] [Google Scholar]; Hughes R. A.; Moody C. J. From amino acids to heteroaromatics—thiopeptide antibiotics, nature’s heterocyclic peptides. Angew. Chem., Int. Ed. 2007, 46 (42), 7930–7954. 10.1002/anie.200700728. [DOI] [PubMed] [Google Scholar]; Katz A. H.; Demerson C. A.; Shaw C. C.; Asselin A. A.; Humber L. G.; Conway K. M.; Gavin G.; Guinosso C.; Jensen N. P. Synthesis and analgesic activity of pemedolac [cis-1-ethyl-1, 3, 4, 9-tetrahydro-4-(phenylmethyl) pyrano [3, 4-b] indole-1-acetic acid]. Journal of medicinal chemistry 1988, 31 (6), 1244–1250. 10.1021/jm00401a029. [DOI] [PubMed] [Google Scholar]; Jiang B.; Gu X.-H. Syntheses of Bis (3′-indolyl)-2 (1H)-pyrazinones. Heterocycles 2000, 53 (7), 1559–1568. 10.3987/COM-00-8887. [DOI] [Google Scholar]; Garg N. K.; Sarpong R.; Stoltz B. M. The first total synthesis of dragmacidin D. J. Am. Chem. Soc. 2002, 124 (44), 13179–13184. 10.1021/ja027822b. [DOI] [PubMed] [Google Scholar]
- Wang X.-W.; Hua Y.-Z.; Wang M.-C. Synthesis of 3-indolylglycine derivatives via dinuclear zinc catalytic asymmetric Friedel–Crafts alkylation reaction. Journal of Organic Chemistry 2016, 81 (19), 9227–9234. 10.1021/acs.joc.6b01805. [DOI] [PubMed] [Google Scholar]
- Hua Y.-Z.; Han X.-W.; Yang X.-C.; Song X.; Wang M.-C.; Chang J.-B. Enantioselective Friedel–Crafts Alkylation of Pyrrole with Chalcones Catalyzed by a Dinuclear Zinc Catalyst. Journal of Organic Chemistry 2014, 79 (23), 11690–11699. 10.1021/jo5023712. [DOI] [PubMed] [Google Scholar]
- Pradere U.; Garnier-Amblard E. C.; Coats S. J.; Amblard F.; Schinazi R. F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114 (18), 9154–9218. 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tang W.; Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003, 103 (8), 3029–3070. 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]
- Shao N.; Luo Y.-Y.; Lu H.-J.; Hua Y.-Z.; Wang M.-C. Enantioselective phospha-Michael reaction of diethyl phosphonate with exocyclic α, β-unsaturated benzocyclic ketones catalyzed by a dinuclear zinc- AzePhenol catalyst. Tetrahedron 2018, 74 (17), 2130–2142. 10.1016/j.tet.2018.03.016. [DOI] [Google Scholar]
- Moskvina V.; Khilya V. Recent Progress in the Synthesis of 4-Arylcoumarins. Chem. Nat. Compd. 2019, 55, 401–427. 10.1007/s10600-019-02705-8. [DOI] [Google Scholar]; Polamreddy P.; Vishwakarma V.; Gundla R. A REVIEW ON ANTI-HCV AGENTS TARGETING ACTIVE SITE AND ALLOSTERIC SITES OF NON-STRUCTURAL PROTEIN 5B [NS5B]. Int. J. Pharm. Pharm. Sci. 2016, 8 (11), 1–18. 10.22159/ijpps.2016v8i11.13965. [DOI] [Google Scholar]; Li X.; Zeng H.; Wang P.; Lin L.; Liu L.; Zhen P.; Fu Y.; Lu P.; Zhu H. Reactivation of latent HIV-1 in latently infected cells by coumarin compounds: Hymecromone and ScoparoneReactivation of Latent HIV-1 in Latently Infected Cells by Coumarin Compounds: Hymecromone and Scoparone. Current HIV research 2016, 14 (6), 484–490. 10.2174/1570162X14666161003152458. [DOI] [PubMed] [Google Scholar]; Iftikhar R.; Zahoor A. F.; Ahmad S.; Haq A. u.; Naheed S. Revisiting the Synthesis of Betti Bases: Facile, One-pot, and Efficient Synthesis of Betti Bases Promoted by FeCl3• 6H2O. Current Organic Synthesis 2022, 19 (5), 569–577. 10.2174/1570179419666220127144352. [DOI] [PubMed] [Google Scholar]
- Liu S.; Xu Z.-H.; Wang X.; Zhu H.-R.; Wang M.-C. Azetidine-derived dinuclear zinc-catalyzed asymmetric conjugate addition of bioactive heterocycles to β, γ-unsaturated α-keto esters. Journal of Organic Chemistry 2019, 84 (21), 13881–13889. 10.1021/acs.joc.9b02046. [DOI] [PubMed] [Google Scholar]
- Coburn C. A.; Meinke P. T.; Chang W.; Fandozzi C. M.; Graham D. J.; Hu B.; Huang Q.; Kargman S.; Kozlowski J.; Liu R.; et al. Discovery of MK-8742: an HCV NS5A inhibitor with broad genotype activity. ChemMedChem. 2013, 8 (12), 1930–1940. 10.1002/cmdc.201300343. [DOI] [PubMed] [Google Scholar]; Li J. J.Heterocyclic chemistry in drug discovery; John Wiley & Sons, 2013. [Google Scholar]; Chavan P. W.; Hanamshetty P. C.; Nagabhushana M.. Biological importance of the indole nucleus in recent years: a comprehensive review. 2010. [Google Scholar]
- Trost B. M.; Gnanamani E.; Hung C. I. Controlling Regioselectivity in the Enantioselective N-Alkylation of Indole Analogues Catalyzed by Dinuclear Zinc-ProPhenol. Angew. Chem. 2017, 129 (35), 10587–10592. 10.1002/ange.201705315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanessian S.Total synthesis of natural products, the Organic chemistry series; Vol. 3, Pergamon Press, 1983. [Google Scholar]; Davis F. A.; Chen B. C. Asymmetric hydroxylation of enolates with N-sulfonyloxaziridines. Chem. Rev. 1992, 92 (5), 919–934. 10.1021/cr00013a008. [DOI] [Google Scholar]; Tanaka T.; Kawase M.; Tani S. α-Hydroxyketones as inhibitors of urease. Bioorganic & medicinal chemistry 2004, 12 (2), 501–505. 10.1016/j.bmc.2003.10.017. [DOI] [PubMed] [Google Scholar]; Wallace O. B.; Smith D. W.; Deshpande M. S.; Polson C.; Felsenstein K. M. Inhibitors of Aβ production: solid-phase synthesis and SAR of α-hydroxycarbonyl derivatives. Bioorganic & medicinal chemistry letters 2003, 13 (6), 1203–1206. 10.1016/S0960-894X(02)01058-2. [DOI] [PubMed] [Google Scholar]; Streuff J. An update on catalytic strategies for the synthesis of α-hydroxyketones. Synlett 2013, 24 (03), 276–280. 10.1055/s-0032-1317716. [DOI] [Google Scholar]
- Gao Y. Y.; Hua Y. Z.; Wang M. C. Asymmetric 1, 6-Conjugate Addition of para-Quinone Methides for the Synthesis of Chiral β, β-Diaryl-α-Hydroxy Ketones. Advanced Synthesis & Catalysis 2018, 360 (1), 80–85. 10.1002/adsc.201700947. [DOI] [Google Scholar]
- Kirsch P.Fluoroorganic chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]; Riether D.; Harcken C.; Razavi H.; Kuzmich D.; Gilmore T.; Bentzien J. r.; Pack E. J. Jr; Souza D.; Nelson R. M.; Kukulka A.; et al. Nonsteroidal dissociated glucocorticoid agonists containing azaindoles as steroid A-ring mimetics. Journal of medicinal chemistry 2010, 53 (18), 6681–6698. 10.1021/jm100751q. [DOI] [PubMed] [Google Scholar]; Sumiyoshi T.; Tojo K.; Urabe D.; Tobe M. Asymmetric synthesis of the 6-cyanoindole derivatives as non-steroidal glucocorticoid receptor modulators using (+)-and (−)-tert-butyl 6-cyano-3-[3-ethoxy-1, 1, 1-trifluoro-2-hydroxy-3-oxopropan-2-yl]-1H-indole-1-carboxylate. Tetrahedron: Asymmetry 2011, 22 (2), 153–160. 10.1016/j.tetasy.2011.01.020. [DOI] [Google Scholar]; Han X.; Ouyang W.; Liu B.; Wang W.; Tien P.; Wu S.; Zhou H.-B. Enantioselective inhibition of reverse transcriptase (RT) of HIV-1 by non-racemic indole-based trifluoropropanoates developed by asymmetric catalysis using recyclable organocatalysts. Organic & Biomolecular Chemistry 2013, 11 (48), 8463–8475. 10.1039/c3ob41667d. [DOI] [PubMed] [Google Scholar]
- Hua Y.-Z.; Chen J.-W.; Yang H.; Wang M.-C. Asymmetric Friedel–Crafts Alkylation of Indoles with Trifluoromethyl Pyruvate Catalyzed by a Dinuclear Zinc Catalyst. Journal of Organic Chemistry 2018, 83 (3), 1160–1166. 10.1021/acs.joc.7b02599. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Saget T.; Lerchen A.; Hung C. I. Catalytic Asymmetric Mannich Reactions with Fluorinated Aromatic Ketones: Efficient Access to Chiral β-Fluoroamines. Angew. Chem., Int. Ed. 2016, 55 (2), 781–784. 10.1002/anie.201509719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini M. α-Amido sulfones as stable precursors of reactive N-acylimino derivatives. Chem. Rev. 2005, 105 (11), 3949–3977. 10.1021/cr050528s. [DOI] [PubMed] [Google Scholar]; Ballini R.; Petrini M. Recent synthetic developments in the nitro to carbonyl conversion (Nef reaction). Tetrahedron 2004, 60 (5), 1017–1047. 10.1016/j.tet.2003.11.016. [DOI] [Google Scholar]; Foresti E.; Palmieri G.; Petrini M.; Profeta R. Highly diastereoselective addition of nitromethane anion to chiral α-amidoalkylphenyl sulfones. Synthesis of optically active α-amino acid derivatives. Organic & biomolecular chemistry 2003, 1 (23), 4275–4281. 10.1039/B309211A. [DOI] [PubMed] [Google Scholar]; Lucet D.; Le Gall T.; Mioskowski C. The chemistry of vicinal diamines. Angew. Chem., Int. Ed. 1998, 37 (19), 2580–2627. . [DOI] [PubMed] [Google Scholar]
- Liu S.; Gao W.-C.; Miao Y.-H.; Wang M.-C. Dinuclear zinc-azephenol catalyzed asymmetric aza-Henry reaction of N-Boc imines and nitroalkanes under ambient conditions. Journal of Organic Chemistry 2019, 84 (5), 2652–2659. 10.1021/acs.joc.8b02943. [DOI] [PubMed] [Google Scholar]
- Kobayashi J. i.; Kubota T. The Daphniphyllum alkaloids. Natural Product Reports 2009, 26 (7), 936–962. 10.1039/b813006j. [DOI] [PubMed] [Google Scholar]; Chrzanowska M.; Rozwadowska M. D. Asymmetric synthesis of isoquinoline alkaloids. Chem. Rev. 2004, 104 (7), 3341–3370. 10.1021/cr030692k. [DOI] [PubMed] [Google Scholar]; Chattopadhyay A. K.; Hanessian S. Recent progress in the chemistry of Daphniphyllum alkaloids. Chem. Rev. 2017, 117 (5), 4104–4146. 10.1021/acs.chemrev.6b00412. [DOI] [PubMed] [Google Scholar]; Ricci A.Modern amination methods; John Wiley & Sons, 2008. [Google Scholar]; Nugent T. C.Chiral amine synthesis: methods, developments and applications; John Wiley & Sons, 2010. [Google Scholar]
- Trost B. M.; Tracy J. S.; Lin E. Y. Asymmetric Electrophilic Amination and Hydrazination of Acyclic α-Branched Ketones for the Formation of α-Tertiary Amines and Hydrazines. ACS Catal. 2019, 9 (12), 11082–11087. 10.1021/acscatal.9b04246. [DOI] [Google Scholar]
- Singh G. S.; Desta Z. Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112 (11), 6104–6155. 10.1021/cr300135y. [DOI] [PubMed] [Google Scholar]; Rios R. Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 2012, 41 (3), 1060–1074. 10.1039/C1CS15156H. [DOI] [PubMed] [Google Scholar]; Xu P.-W.; Yu J.-S.; Chen C.; Cao Z.-Y.; Zhou F.; Zhou J. Catalytic enantioselective construction of spiro quaternary carbon stereocenters. ACS Catal. 2019, 9 (3), 1820–1882. 10.1021/acscatal.8b03694. [DOI] [Google Scholar]
- Cao Z.-Y.; Zhou F.; Zhou J. Development of synthetic methodologies via catalytic enantioselective synthesis of 3, 3-disubstituted oxindoles. Acc. Chem. Res. 2018, 51 (6), 1443–1454. 10.1021/acs.accounts.8b00097. [DOI] [PubMed] [Google Scholar]
- Franz A. K.; Dreyfuss P. D.; Schreiber S. L. Synthesis and cellular profiling of diverse organosilicon small molecules. J. Am. Chem. Soc. 2007, 129 (5), 1020–1021. 10.1021/ja067552n. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.; Chafeev M.; Liu S.; Sun J.; Raina V.; Chui R.; Young W.; Kwan R.; Fu J.; Cadieux J. A. Discovery of XEN907, a spirooxindole blocker of NaV1. 7 for the treatment of pain. Bioorganic & medicinal chemistry letters 2011, 21 (12), 3676–3681. 10.1016/j.bmcl.2011.04.088. [DOI] [PubMed] [Google Scholar]
- Guo Y.-J.; Guo X.; Kong D.-Z.; Lu H.-J.; Liu L.-T.; Hua Y.-Z.; Wang M.-C. Catalytic asymmetric synthesis of tetrahydrofuran spirooxindoles via a dinuclear zinc catalyst. Journal of Organic Chemistry 2020, 85 (6), 4195–4206. 10.1021/acs.joc.9b03378. [DOI] [PubMed] [Google Scholar]
- Bartoli A.; Rodier F.; Commeiras L.; Parrain J.-L.; Chouraqui G. Construction of spirolactones with concomitant formation of the fused quaternary centre–application to the synthesis of natural products. Natural product reports 2011, 28 (4), 763–782. 10.1039/c0np00053a. [DOI] [PubMed] [Google Scholar]; Mao B.; Fañanás-Mastral M.; Feringa B. L. Catalytic asymmetric synthesis of butenolides and butyrolactones. Chem. Rev. 2017, 117 (15), 10502–10566. 10.1021/acs.chemrev.7b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kitson R. R.; Millemaggi A.; Taylor R. J. The renaissance of α-methylene-γ-butyrolactones: new synthetic approaches. Angew. Chem., Int. Ed. 2009, 48 (50), 9426–9451. 10.1002/anie.200903108. [DOI] [PubMed] [Google Scholar]; Lepoittevin J. P.; Berl V.; Giménez-Arnau E. α-methylene-γ-butyrolactones: versatile skin bioactive natural products. Chem. Rec. 2009, 9 (5), 258–270. 10.1002/tcr.200900013. [DOI] [PubMed] [Google Scholar]
- Quintavalla A. Spirolactones: Recent advances in natural products, bioactive compounds and synthetic strategies. Curr. Med. Chem. 2018, 25 (8), 917–962. 10.2174/0929867324666171106162259. [DOI] [PubMed] [Google Scholar]; Kolkhof P.; Bärfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. Journal of endocrinology 2017, 234 (1), T125. 10.1530/JOE-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pitt B.; Zannad F.; Remme W. J.; Cody R.; Castaigne A.; Perez A.; Palensky J.; Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. New England Journal of Medicine 1999, 341 (10), 709–717. 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]; Elger W.; Beier S.; Pollow K.; Garfield R.; Shi S. Q.; Hillisch A. Conception and pharmacodynamic profile of drospirenone. Steroids 2003, 68 (10–13), 891–905. 10.1016/j.steroids.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Liu M.-M.; Yang X.-C.; Hua Y.-Z.; Chang J.-B.; Wang M.-C. Dinuclear zinc-catalyzed asymmetric tandem reaction of α-hydroxy-1-indanone: access to spiro [1-indanone-5, 2′-γ-butyrolactones]. Org. Lett. 2019, 21 (17), 7089–7093. 10.1021/acs.orglett.9b02658. [DOI] [PubMed] [Google Scholar]
- Saeed A.; Haroon M.; Muhammad F.; Larik F. A.; Hesham E.-S.; Channar P. A. Advances in transition-metal-catalyzed synthesis of 3-substituted isocoumarins. J. Organomet. Chem. 2017, 834, 88–103. 10.1016/j.jorganchem.2017.02.016. [DOI] [Google Scholar]; Issaian A.; Tu K. N.; Blum S. A. Boron–heteroatom addition reactions via borylative heterocyclization: oxyboration, aminoboration, and thioboration. Acc. Chem. Res. 2017, 50 (10), 2598–2609. 10.1021/acs.accounts.7b00365. [DOI] [PubMed] [Google Scholar]; Chaudhary N. K.; Pitt J. I.; Lacey E.; Crombie A.; Vuong D.; Piggott A. M.; Karuso P. Banksialactones and banksiamarins: isochromanones and isocoumarins from an Australian fungus, Aspergillus banksianus. J. Nat. Prod. 2018, 81 (7), 1517–1526. 10.1021/acs.jnatprod.7b00816. [DOI] [PubMed] [Google Scholar]; Shimojima Y.; Shirai T.; Baba T.; Hayashi H. 1H-2-Benzopyran-1-one derivatives, microbial products with pharmacological activity. Conversion into orally active derivatives with antiinflammatory and antiulcer activities. Journal of medicinal chemistry 1985, 28 (1), 3–9. 10.1021/jm00379a002. [DOI] [PubMed] [Google Scholar]; Endringer D. C.; Guimarães K. G.; Kondratyuk T. P.; Pezzuto J. M.; Braga F. C. Selective inhibition of aromatase by a dihydroisocoumarin from Xyris pterygoblephara. J. Nat. Prod. 2008, 71 (6), 1082–1084. 10.1021/np800098f. [DOI] [PMC free article] [PubMed] [Google Scholar]; Saeed A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. 10.1016/j.ejmech.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Yang X.-C.; Xu M.; Wang J.-B.; Liu M.-M.; Mathey F.; Hua Y.-Z.; Wang M.-C. Enantioselective synthesis of indanone spiro-isochromanone derivatives via a dinuclear zinc-catalyzed Michael/transesterification tandem reaction. Organic & Biomolecular Chemistry 2020, 18 (20), 3917–3926. 10.1039/D0OB00541J. [DOI] [PubMed] [Google Scholar]
- Pellissier H. Enantioselective magnesium-catalyzed transformations. Organic & Biomolecular Chemistry 2017, 15 (22), 4750–4782. 10.1039/C7OB00903H. [DOI] [PubMed] [Google Scholar]; Pellissier H. Stereocontrolled domino reactions. Chem. Rev. 2013, 113 (1), 442–524. 10.1021/cr300271k. [DOI] [PubMed] [Google Scholar]; Pellissier H. Recent developments in asymmetric organocatalytic domino reactions. Advanced Synthesis & Catalysis 2012, 354 (2–3), 237–294. 10.1002/adsc.201100714. [DOI] [Google Scholar]; Chanda T.; Zhao J. C. G. Recent progress in organocatalytic asymmetric domino transformations. Advanced Synthesis & Catalysis 2018, 360 (1), 2–79. 10.1002/adsc.201701059. [DOI] [Google Scholar]; Yu C.; Lyu H.; Cai Y.; Miao X.; Miao Z. An environmentally benign approach for the synthesis of 3, 3′-pyrrolidonyl spirooxindole derivatives via a cascade Knoevenagel–Michael–cyclization multicomponent reaction. RSC Adv. 2013, 3 (41), 18857–18862. 10.1039/c3ra44119a. [DOI] [Google Scholar]; Zhu Q.-N.; Zhang Y.-C.; Xu M.-M.; Sun X.-X.; Yang X.; Shi F. Enantioselective construction of tetrahydroquinolin-5-one-based spirooxindole scaffold via an organocatalytic asymmetric multicomponent [3+ 3] cyclization. Journal of organic chemistry 2016, 81 (17), 7898–7907. 10.1021/acs.joc.6b01598. [DOI] [PubMed] [Google Scholar]; Yu D.-F.; Wang Y.; Xu P.-F. Organocatalytic enantioselective multicomponent cascade reaction: facile access to tetrahydropyridines with C3 all-carbon quaternary stereocenters. Tetrahedron 2011, 67 (18), 3273–3277. 10.1016/j.tet.2011.02.047. [DOI] [Google Scholar]
- Miao Y.-H.; Hua Y.-Z.; Wang M.-C. Dinuclear zinc cooperative catalytic three-component reactions for highly enantioselective synthesis of 3, 3′-dihydrofuran spirooxindoles. Organic & Biomolecular Chemistry 2019, 17 (30), 7172–7181. 10.1039/C9OB01233H. [DOI] [PubMed] [Google Scholar]
- Liu T.-T.; Chen Y.; Mei G.-J.; Hua Y.-Z.; Jia S.-K.; Wang M.-C. Zinc-Catalyzed Enantioselective [3+ 3] Annulation for Synthesis of Chiral Spiro [indoline-3, 4′-thiopyrano [2, 3-b] indole] Derivatives. Molecules 2023, 28 (3), 1056. 10.3390/molecules28031056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-C.; Liu M.-M; Mathey F. o.; Yang H.; Hua Y.-Z.; Wang M.-C. Access to Chiral 2,5-Pyrrolidinyl Dispirooxindoles via Dinuclear Zinc-Catalyzed Asymmetric Cascade Reactions. The Journal of Organic Chemistry 2019, 84, 7762–7775. 10.1021/acs.joc.9b00645. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Liu Y. L.; Zhou J. Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Advanced Synthesis & Catalysis 2010, 352, 1381–1407. 10.1002/adsc.201000161. [DOI] [Google Scholar]
- Mei G.-J.; Shi F. Catalytic asymmetric synthesis of spirooxindoles: recent developments. Chemical Communications 2018, 54, 6607–6621. 10.1039/C8CC02364F. [DOI] [PubMed] [Google Scholar]
- Kumar R. R.; Perumal S.; Senthilkumar P.; Yogeeswari P.; Sriram D. A facile synthesis and antimycobacterial evaluation of novel spiro-pyrido-pyrrolizines and pyrrolidines. European journal of medicinal chemistry 2009, 44, 3821–3829. 10.1016/j.ejmech.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Tan W.; Zhu X.-T.; Zhang S.; Xing G.-J.; Zhu R.-Y.; Shi F. Diversity-oriented synthesis of spiro-oxindole-based 2,5-dihydropyrroles via three-component cycloadditions and evaluation on their cytotoxicity. RSC advances 2013, 3, 10875–10886. 10.1039/c3ra40874d. [DOI] [Google Scholar]
- Yang X.-C.; Liu M.-M.; Mathey F. o.; Yang H.; Hua Y.-Z.; Wang M.-C. Access to chiral 2, 5-pyrrolidinyl dispirooxindoles via dinuclear zinc-catalyzed asymmetric cascade reactions. Journal of Organic Chemistry 2019, 84 (12), 7762–7775. 10.1021/acs.joc.9b00645. [DOI] [PubMed] [Google Scholar]
- Hong Hu; Hollinshead S. P.; Hall S. E.; Kalter K.; Ballas L. M. Synthesis and protein kinase C inhibitory activities of Indane analogs of balanol. Bioorg. Med. Chem. Lett. 1996, 6 (8), 973–978. 10.1016/0960-894X(96)00151-5. [DOI] [Google Scholar]; Gross M. F.; Beaudoin S.; McNaughton-Smith G.; Amato G. S.; Castle N. A.; Huang C.; Zou A.; Yu W. Aryl sulfonamido Indane inhibitors of the Kv1. 5 ion channel. Bioorganic & medicinal chemistry letters 2007, 17 (10), 2849–2853. 10.1016/j.bmcl.2007.02.052. [DOI] [PubMed] [Google Scholar]; Ulmschneider S.; Müller-Vieira U.; Klein C. D.; Antes I.; Lengauer T.; Hartmann R. W. Synthesis and evaluation of (pyridylmethylene) tetrahydronaphthalenes/-indanes and structurally modified derivatives: potent and selective inhibitors of aldosterone synthase. Journal of medicinal chemistry 2005, 48 (5), 1563–1575. 10.1021/jm0492397. [DOI] [PubMed] [Google Scholar]; Hudson S.; Kiankarimi M.; Eccles W.; Mostofi Y. S.; Genicot M. J.; Dwight W.; Fleck B. A.; Gogas K.; Wade W. S. Synthesis and structure–activity relationships of selective norepinephrine reuptake inhibitors (sNRI) with a heterocyclic ring constraint. Bioorganic & medicinal chemistry letters 2008, 18 (16), 4495–4498. 10.1016/j.bmcl.2008.07.050. [DOI] [PubMed] [Google Scholar]; Clark W. M.; Tickner-Eldridge A. M.; Huang G. K.; Pridgen L. N.; Olsen M. A.; Mills R. J.; Lantos I.; Baine N. H. A catalytic enantioselective synthesis of the endothelin receptor antagonists SB-209670 and SB-217242. A base-catalyzed stereospecific formal 1, 3-hydrogen transfer of a chiral 3-arylindenol. J. Am. Chem. Soc. 1998, 120 (18), 4550–4551. 10.1021/ja973882j. [DOI] [Google Scholar]
- Tao B.-K.; Yang H.; Hua Y.-Z.; Wang M.-C. Dinuclear zinc synergistic catalytic asymmetric phospha-Michael/Michael cascade reaction: synthesis of 1, 2, 3-trisubstituted indanes bearing phosphoryl groups. Organic & Biomolecular Chemistry 2019, 17 (17), 4301–4310. 10.1039/C9OB00544G. [DOI] [PubMed] [Google Scholar]
- Liu M.-M.; Yang X.-C.; Hua Y.-Z.; Chang J.-B.; Wang M.-C. Synthesis of chiral bispirotetrahydrofuran oxindoles by cooperative bimetallic-catalyzed asymmetric Cascade Reaction. Org. Lett. 2019, 21 (7), 2111–2115. 10.1021/acs.orglett.9b00386. [DOI] [PubMed] [Google Scholar]
- Jensen R.; Rasmussen B. K. Burden of headache. Expert Review of Pharmacoeconomics & Outcomes Research 2004, 4 (3), 353–359. 10.1586/14737167.4.3.353. [DOI] [PubMed] [Google Scholar]; Galliford C. V.; Scheidt K. A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem., Int. Ed. 2007, 46 (46), 8748–8758. 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]; Trost B. M.; Brennan M. K. Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis 2009, 2009 (18), 3003–3025. 10.1055/s-0029-1216975. [DOI] [Google Scholar]
- Trost B. M.; Gnanamani E.; Kalnmals C. A.; Hung C.-I. J.; Tracy J. S. Direct enantio-and diastereoselective vinylogous addition of butenolides to chromones catalyzed by Zn-prophenol. J. Am. Chem. Soc. 2019, 141 (4), 1489–1493. 10.1021/jacs.8b13367. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Krohn K.; Zia-Ullah; Florke U.; Pescitelli G.; Di Bari L.; Antus S.; Kurtan T.; Rheinheimer J.; Draeger S.; Schulz B. New Mono-and Dimeric Members of the Secalonic Acid Family: Blennolides A–G Isolated from the Fungus Blennoria sp. Chemistry–A European Journal 2008, 14 (16), 4913–4923. 10.1002/chem.200800035. [DOI] [PubMed] [Google Scholar]
- Xing S.-N.; Hua Y.-Z.; Yang X.-C.; Du S.-S.; Jia S.-K.; Mei G.-J.; Wang M.-C. Catalytic Asymmetric Umpolung Tandem Reactions of Hemiacetals via Dinuclear Zinc Cooperative Catalysis. Org. Lett. 2022, 24 (22), 3909–3914. 10.1021/acs.orglett.2c00913. [DOI] [PubMed] [Google Scholar]