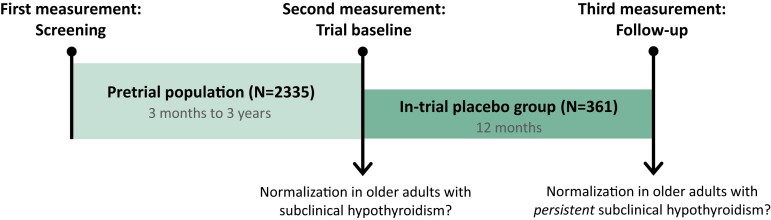
Figure 1.
Timeline of the study. The pretrial population consisted of older adults with ≥1 elevated TSH measurement (≥4.60 mIU/L) and an fT4 level within the laboratory-specific reference range, during the previous 3 months to 3 years (screening). The second measurement was at the trial baseline to assess whether they had persistent subclinical hypothyroidism or had normalized their TSH levels. The in-trial placebo group consisted of older adults with ≥2 elevated TSH measurements, both at the screening and trial baseline, receiving placebo in the clinical trials. After 12 months, at the (third) follow-up measurement, it was determined whether the in-trial placebo group had normalized their TSH levels.