Abstract
A 53-year-old woman presented with a 2-week history of headache and vertigo. Computed tomography revealed a hyperdense tumor, measuring 30 × 31 × 36 mm in diameter, in the anteromedial parts of the cerebellar hemispheres. Cerebral magnetic resonance imaging 10 days later revealed an apparent extra-axial tumor with broad attachment to the medial tentorium cerebelli and rapid growth to a diameter of 40 × 41 × 46 mm. Cerebral angiography revealed no obvious feeding vessels or tumor stains. The patient underwent biopsy through the left occipital transtentorial route. The histological appearance was consistent with diffuse large B-cell lymphoma. Intracranial lymphoma may present as a dural tumor that mimics a meningioma. Rapid tumor growth incongruous with benign meningiomas should be assumed to be possible lymphoma, and prompt biopsy should be performed.
Keywords: Lymphoma, Dural-based, Mimic meningioma, Occipital transtentorial approach
Introduction
Meningiomas are frequent and typically benign primary brain tumors in adulthood. Tentorial meningiomas are rare, but distinct entities. They are located deep in the cranial cavity, attaching to the tentorium cerebelli, and adjacent to essential venous structures. These tumors commonly present with nonspecific symptoms and remain asymptomatic until they reach a considerably large size [1,2]. These tumors are frequently resected via the occipital transtentorial route [3].
Variable pathologies involving tumorous and non-tumorous lesions can mimic meningioma on radiological examination [4], [5], [6], [7]. Dural-based lymphomas as mimics of meningiomas have been rarely documented [8], [9], [10], [11], [12], [13]. A recent investigation proposed that multi-marker algorithms using biomarkers in cerebrospinal fluid (CSF) could be useful for diagnosing central nervous system lymphoma [14].
Here, we present a unique case of dural large B-cell lymphoma mimicking tentorial meningioma.
Case report
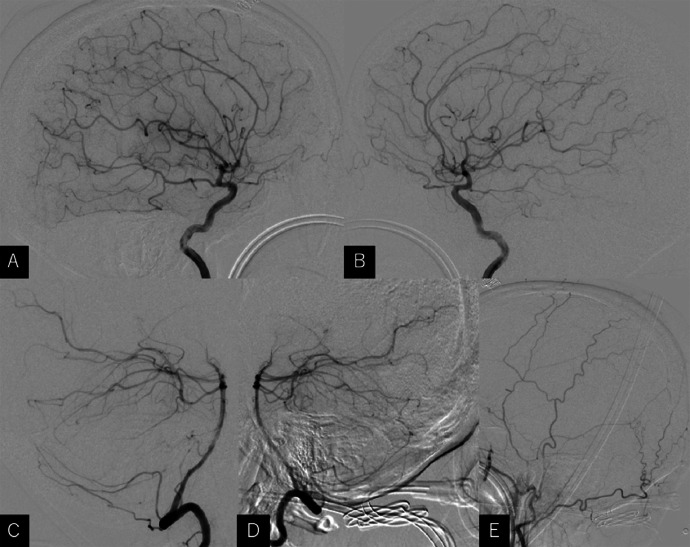
A previously healthy 53-year-old woman presented with a 2-week history of headache and vertigo. At presentation, the patient was well-oriented but showed mild truncal ataxia and gait unsteadiness. Nystagmus was not observed. Computed tomography (CT) revealed a hyperdense tumor measuring 30 × 31 × 36 mm in diameter, located in the anteromedial parts of the cerebellar hemispheres, and mild ventriculomegaly. On contrast examination, the tumor was located adjacent to the lower surface of the medial tentorium cerebelli, and between the vein of cerebellomesencephalic fissure and the declival vein. It also compressed the dorsal brainstem resulting in a stenosis of the aqueduct (Fig. 1). With a presumptive diagnosis of tentorial meningioma, the patient was scheduled to undergo cerebral magnetic resonance imaging (MRI) 10 days later, when her symptoms exacerbated and she was unable to walk without assistance. The MRI revealed an apparent extra-axial tumor with broad attachments to the medial tentorium cerebelli, predominantly on the left side. The tumor appeared as hypointensity on T1- and mixed intensity on T2- and diffusion-weighted (DWI) sequences, accompanied by extensive perilesional brain edema (Figs. 2A–C). Apparent diffusion coefficient map of the DWI showed hypointensity. On contrast examination using gadolinium, it was well-demarcated, measuring 40 × 41 × 46 mm in diameter, and was intensely enhanced (Figs. 2D–F). With the presumptive diagnosis of tentorial meningioma, the patient underwent cerebral angiography. Unexpectedly, it detected no obvious feeding vessels or tumor stains in the internal carotid, external carotid, or vertebrobasilar systems (Fig. 3). Then, we assumed lymphoma as differential diagnosis. At that time, the serum level of soluble interleukin-2 receptor (sIL-2R) was 470 U/mL (normal range, 157-474). Given the potentially malignant nature of the rapidly growing tumor, the patient underwent biopsy through the left occipital transtentorial route. The paramedian tentorial dura was incised in the anteroposterior direction, parallel to the straight sinus, that exposed the tumor lying just below. The tumor was grayish, elastic, soft, and less vascular. Adhesions between the tumor and the tentorium cerebelli were not observed. The resected specimens showed sheet-like proliferation of anaplastic cells with a high nuclear-to-cytoplasmic ratio and pleomorphism (Fig. 4A). Immunohistochemical examination showed positive staining for CD20 (Fig. 4B), but negative staining for CD3, AE1/3, and glial fibrillary acidic protein. These findings suggested diffuse large B-cell lymphoma. A systematic survey performed postoperatively did not identify any foci of extracranial lymphoma. Chemotherapy using rituximab, methotrexate, vincristine, and procarbazine resulted in a remarkable improvement in the patient's symptoms. Cranial CT 10 days after beginning of the chemotherapy showed a marked regression of the tumor (Fig. 5).
Fig. 1.
Noncontrast axial computed tomography (CT) scan showing a hyperdense tumor (T) in the anteromedial parts of the cerebellar hemispheres and mild ventriculomegaly (A). Post-contrast coronal (B) and sagittal (C) CT scans showing that the tumor, measuring 30 × 31 × 36 mm, is located in the posterior fossa, adjacent to the lower surface of medial tentorium cerebelli (TCe) and between the vein of the cerebellomesencephalic fissure (*) and declival vein (**), and compresses the dorsal brainstem, resulting in stenosis of the aqueduct.
Fig. 2.
Axial T1- (A), T2- (B), and diffusion-weighted (C) magnetic resonance imaging show the tumor appearing as hypointensity on T1- and mixed intensity on T2- and diffusion-weighted sequences, respectively, accompanied by an extensive perilesional brain edema (B). On contrast examination, the tumor is well-demarcated, measuring 40 × 41 × 46 mm, and intensely enhanced (D–F).
Fig. 3.
Lateral views of the right (A) and left internal (B), right (C) and left vertebral (D), and left external carotid arteriography (E) detecting no obvious feeding vessels from the internal carotid, vertebrobasilar, or external carotid system.
Fig. 4.
Photomicrographs of resected specimens showing sheet-like proliferation of anaplastic cells with a high nuclear-cytoplasmic ratio and pleomorphism (A, hematoxylin, and eosin stain). These cells are positively stained for CD20 (B). Original magnification of A and B, × 400.
Fig. 5.
Noncontrast axial (A) and sagittal (B) computed tomography scans performed 10 days after the beginning of chemotherapy showed a marked regression of the tumor (T) and cerebral ventricles with patent aqueduct.
Discussion
Various pathologies can mimic meningiomas on neuroimaging. In addition to lymphomas, solitary fibrous tumors/hemangiopericytomas, gliosarcomas, glioblastomas, leiomyosarcomas, melanocytomas, Hodgkin's disease, plasmacytomas, inflammatory pseudotumors, neurosarcoidosis, plasma cell granulomas, Rosai-Dorfman disease, Castleman disease, xanthomas, rheumatoid nodules, and tuberculomas mimic meningioma [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. To the best of our knowledge, only 1 case of dural-based lymphoma mimicking tentorial meningioma has been documented. In the case, a 26-year-old man presented with headache and visual field impairment due to development of a dural-based tumor on the supratentorial side [8]. In our case, the presumptive diagnosis was tentorial meningioma based on the appearance of CT. However, doubts were raised on the MRI examination performed 10 days later, which revealed a rapid tumor growth. Furthermore, cerebral angiography did not detect any feeding vessels via the meningeal or tentorial arteries, atypical of tentorial meningiomas. Then, we suspected a malignant tumor and performed biopsy.
In the present case, the serum level of sIL-2R, a non-specific biomarker of lymphoma, was within the normal range. If safely carried out, CSF examination targeted at CXCL13, IL-10, sIL-2R, and β2 microglobulin could be useful to diagnose intracranial lymphoma [14].
The occipital transtentorial approach is thought to be useful for resecting meningiomas of the tentorial notch and pineal region [2,3]. In contrast, due to limited exposure, the approach is unsuitable for large meningiomas with significant contralateral infratentorial extension [3]. In the present case, the offending tumor was large and located immediately below the tentorial cerebelli on both sides. In addition, the posterior cranial fossa appeared highly crowded on pre-surgical MRI, anticipating a high risk when approaching the tumor through an infratentorial route. Furthermore, the purpose of surgery was biopsy, instead of radical tumor resection. Therefore, in the present case, the unilateral occipital transtentorial route was adopted.
Intracranial lymphoma may present as a dural tumor that mimics a meningioma. Rapid tumor growth incongruous with benign meningiomas should be assumed to be possible lymphoma, and prompt biopsy should be performed.
Patient consent
The authors certify that they have obtained appropriate patient consent to publish this case report.
Author contributions
All the authors contributed equally to the study.
Ethical standards
We declare that all procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments.
Patient consent
The patients documented in the manuscript fully understood and agreed that the authors use the information materials of the patients in anonymized manner for possible publication in Radiology Case Reports.
Footnotes
Competing Interests: The authors declare that they have no known conflicts of interest, competing financial interests, or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bassiouni H, Hunold A, Asgari S, Stolke D. Tentorial meningiomas: clinical results in 81 patients treated microsurgically. Neurosurgery. 2004;55(1):116–118. doi: 10.1227/01.neu.0000126886.48372.49. [DOI] [PubMed] [Google Scholar]
- 2.Samii M, Carvalho GA, Tatagiba M, Matthies C, Vorkapic P. Meningiomas of the tentorial notch: surgical anatomy and management. J Neurosurg. 1996;84(3):375–381. doi: 10.3171/jns.1996.84.3.0375. [DOI] [PubMed] [Google Scholar]
- 3.Qiu B, Wang Y, Ou S, Guo Z, Wang Y. The unilateral occipital transtentorial approach for pineal region meningioma: a report of 15 cases. Int J Neurosci. 2014;124(10):741–747. doi: 10.3109/00207454.2013.878341. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33(12):1211–1226. doi: 10.1053/hupa.2002.129200. [DOI] [PubMed] [Google Scholar]
- 5.Joshi SS, Joshi S, Muzumdar G, Turel KE, Shah RM, Ammbulkar I, et al. Cranio-spinal Rosai Dorfman disease: case series and literature review. Br J Neurosurg. 2019;33(2):176–183. doi: 10.1080/02688697.2017.1329517. [DOI] [PubMed] [Google Scholar]
- 6.Nagai Yamaki V, de Souza Godoy LF, Alencar Bandeira G, Tavares Lucato L, Correa Lordelo G, Fontoura Solla DJ, et al. Dural-based lesions: is it a meningioma? Neuroradiology. 2021;63(8):1215–1225. doi: 10.1007/s00234-021-02632-y. [DOI] [PubMed] [Google Scholar]
- 7.Patel M, Nguyen HS, Doan N, Gelsomino M, Shabani S, Mueller W. Glioblastoma mimicking meningioma: report of 2 cases. World Neurosurg. 2016;95:624. doi: 10.1016/j.wneu.2016.08.048. e9-13. [DOI] [PubMed] [Google Scholar]
- 8.Hirano Y, Miyawaki S, Satou M, Taoka K, Toyama K, Ikemura M, et al. Small cell variant of anaplastic lymphoma kinase-positive anaplastic large cell lymphoma of the dura mimicking tentorial meningioma. World Neurosurg. 2020;138:169–173. doi: 10.1016/j.wneu.2020.02.171. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni KM, Sternau L, Dubovy SR, Lam BL. Primary dural lymphoma masquerading as a meningioma. J Neuroophthalmol. 2012;32(3):240–242. doi: 10.1097/WNO.0b013e31825103a5. [DOI] [PubMed] [Google Scholar]
- 10.Low I, Allen J. Low-grade follicular lymphoma in the dura: rare mimic of meningioma. Neuropathology. 2006;26(6):564–568. doi: 10.1111/j.1440-1789.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 11.Quinn ZL, Zakharia K, Schmid JL, Schmieg JJ, Safah H, Saba NS. Primary dural diffuse large B-cell lymphoma: a comprehensive review of survival and treatment outcomes. Clin Lymphoma Melanoma Leuk. 2020;20(2):e105–e112. doi: 10.1016/j.clml.2019.09.600. [DOI] [PubMed] [Google Scholar]
- 12.Saidy J, Bertal A, Hmada S, Aamara N, Tahrir Y, Mokhliss S, et al. Primary dural lymphoma: case report. Ann Med Sur (Lond) 2022;79 doi: 10.1016/j.amsu.2022.103984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreenivasan S, Solanki R, Kancharla P, Khan C, Samhouri Y. A meningioma mimic and distinct subtype of primary central nervous system lymphoma: primary dural lymphoma. J Hematol. 2023;12(2):87–91. doi: 10.14740/jh1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeyama M, Sasayama T, Tanaka K, Nakamizo S, Tanaka H, Nishihara M, et al. Multi-marker algorithms based on CXCL13, IL-10, sIL-2 receptor, andβ2-microglobulin in cerebrospinal fluid to diagnose CNS lymphoma. Cancer Med. 2020;9(12):4114–4125. doi: 10.1002/cam4.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]