Abstract
Recent studies have demonstrated that in addition to their antimicrobial activity, cationic host defense peptides, like the human cathelicidin LL-37, perform many activities relating to innate immunity, including the induction or modulation of chemokine and cytokine production, alteration of gene expression in host cells, and inhibition of proinflammatory responses of host cells to bacterial components such as lipopolysaccharide (LPS) in vitro and in vivo. To investigate if these properties are shared by smaller peptides, two cathelicidin peptides derived from bovine neutrophils, the 13-mer indolicidin and Bac2A, a linear 12-amino-acid derivative of bactenecin, were compared to the 37-amino-acid peptide LL-37. Indolicidin, like LL-37, inhibited LPS-induced tumor necrosis factor alpha (TNF-α) secretion, even when added up to an hour after the addition of Escherichia coli O111:B4 LPS to the human macrophage/monocyte-like THP-1 cell line. In contrast, Bac2A demonstrated no significant antiendotoxin activity. At low concentrations, indolicidin and LL-37 acted synergistically to suppress LPS-induced production of TNF-α. Indolicidin was analogous to LL-37 in its ability to induce the production of the chemokine interleukin-8 (IL-8) in a human bronchial cell line, 16HBE14o−, but it was unable to induce production of IL-8 in THP-1 cells. In contrast, Bac2A was unable to induce IL-8 in either cell type. Conversely, Bac2A was chemotactic for THP-1 cells at concentrations between 10 and 100 μg/ml, while indolicidin and LL-37 were not chemotactic at these concentrations for THP-1 cells. This indicates that in addition to the potential for direct microbicidal activity, cationic host defense peptides may have diverse and complementary abilities to modulate the innate immune response.
Cationic antimicrobial peptides are conserved across virtually all forms of life as a primitive component of the innate immune response. They can be expressed either constitutively or in response to pathogen-associated molecular pattern molecules, such as bacterial lipopolysaccharide (LPS), or inflammatory mediators, such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) (6, 41). Although they can be potent antimicrobial agents, a key element of their therapeutic potential may involve the myriad of other activities attributed to them (25). Indeed, some peptides such as the human cathelicidin LL-37 have been proposed to have far more potent immunomodulatory activities than antimicrobial functions (1, 24). When considering the use of peptides like LL-37 in immunotherapy, one must take into account the large size of this peptide and the corresponding issues this raises, including cost of goods, protease lability, and pharmacokinetics. In this study, we investigated the immunomodulatory properties of two of the smallest known active peptides, both derived from bovine cathelicidins, and contrasted those activities to LL-37, a known immunomodulator, with the goal of developing novel immunomodulatory therapies.
Naturally occurring cationic peptides can vary in size from 12 to 50 amino acids and have the property of folding into amphipathic structures (often after contact with membranes) that have a positively charged hydrophilic face and a hydrophobic face. In humans, the major linear peptide is the sole cathelicidin characterized to date, LL-37. LL-37 is the proteolytically processed extracellular form of hCAP-18, a cathelicidin peptide which is constitutively produced in the secondary granules of neutrophils and by a variety of other cells. Although found at mucosal surfaces at concentrations of around 2 μg/ml, its expression is induced upon exposure to proinflammatory mediators or during the course of infection or inflammation in a variety of tissues (1, 14, 20, 36). Although cathelicidins are not well conserved between species, the evolutionary relationship between these peptides can be inferred from the highly conserved proregion called the cathelin domain that is cleaved to release the active peptide. Cathelicidins have been found in cows (BMAP-27, indolicidin, and bactenecin), pigs (protegrins), mice (CRAMP), rabbits (CAP18) and humans (hCAP-18/LL-37), and this evolutionary conservation suggests an important role in innate immunity (reviewed in reference 40). To date, studies of the influence of peptides as effectors of innate immunity have tended to utilize larger peptides of 26 amino acids or more in size (9). In this study, two of the shortest known peptides, indolicidin and Bac2A, a derivative of bactenecin, were investigated for their ability to affect a variety of innate immune responses such as cytokine production, antiendotoxin activity, and chemotaxis. Indolicidin, a 13-amino-acid, proline- and tryptophan-rich cathelicidin, folds into a characteristic boat-shaped structure when associated with membranes (23). Its moderate antimicrobial activity (MICs of between 16 and 64 μg/ml for common gram-negative bacteria and between 4 and 8 μg/ml for gram-positive bacteria) and its ability to interact with microbial membranes have been well characterized (7). Bactenecin is a 12-amino-acid cathelicidin that is also moderately active against many common gram-negative pathogens (MICs of approximately 8 μg/ml) and gram-positive bacteria (MICs of 64 μg/ml or greater). The functional structure of bactenecin in vivo has not been well characterized, and there is some evidence for a linear structure (38). Bac2A is a linearized derivative of bactenecin in which two cysteine residues have been replaced with two alanine residues. It has modest broad-spectrum activity, with MICs of between 2 and 32 μg/ml for gram-negative bacteria and between 0.25 and 16 μg/ml for gram-positive bacteria, that is consistent with the activity of the disulfide-bonded peptide (22). This modification prevents the possibility of concatemer formation, thus making Bac2A a better target for drug development.
Previous research has shown that LL-37 is a potent immunomodulator. LL-37 has been demonstrated to be a chemoattractant for human monocytes, T cells (5), and mast cells (18); is a potent antiendotoxic agent (27); and induces chemokine production (24). In light of these observations, we compared the immunomodulatory activities of the two bovine cathelicidins, indolicidin and Bac2A, to those of the better-characterized LL-37 with respect to antiendotoxin properties, chemotaxis, and chemokine production. We found that indolicidin and Bac2A have different immunomodulatory properties, which make them candidates for development as novel therapies.
MATERIALS AND METHODS
Cell lines and culture conditions.
The human monocyte-like cell line THP-1 (33) was obtained from the American Type Culture Collection (Rockville, MD) (ATCC TIB-202) and grown in supplemented RPMI 1640 medium containing 10% fetal calf serum and 1% l-glutamine (Gibco BRL, Burlington, Ontario, Canada). THP-1 cells were differentiated into adherent macrophage-like cells by the addition of 100 mM phorbol myristate acetate and incubation at 37°C in 5% CO2 for 3 days as described previously (32). Undifferentiated THP-1 monocytes were used for the chemotaxis experiments. Differentiated macrophage-like cells were used for the antiendotoxin assays. The human bronchial epithelial cell line 16HBE14o− (HBE) was a gift from D. Gruenert (Cardiovascular Research Institute, University of California) (4) and was maintained in minimal essential medium (MEM) with Earle's salts (Gibco BRL, Burlington, Ontario, Canada). HBE cells were maintained in flasks and seeded into wells that had been treated with mouse type 4 collagen and human fibronectin solution (8.5 ml minimal essential medium with Earle's salts, 100 μl fibronectin, 100 μl collagen, and 1 ml of 1 mg/ml bovine serum albumin; Gibco BRL, Burlington, Ontario, Canada).
Peptide synthesis.
All peptides were synthesized by N-(9-fluorenyl) methoxycarbonyl chemistry at the Nucleic Acid/Protein Service unit at the University of British Columbia, as previously described (10). Peptides were purified by reverse-phase high-performance liquid chromatography and were at least 98% pure. The concentration of the peptides in solution was determined by amino acid analysis.
Cytotoxicity assay.
An MTT assay (American Type Culture Collection) was used to assess the cytotoxicity of the peptides and was performed as per the manufacturer's instructions. The assay is based on the cleavage of the yellow tetrazolium salt MTT into purple formazan by metabolically active cells. Cells were cultured as described above, and peptides were added for 24 h. A decrease in the absorbance at 550 nm indicated a decrease in cell viability.
Cytokine production.
IL-8 or TNF-α found in the supernatants of the treated cells was measured using commercially prepared enzyme-linked immunosorbent assay (ELISA) plates in accordance with the manufacturer's suggestion (R&D systems, Minneapolis, MN). Cells were seeded in 24-well tissue culture plates at a concentration of 2 × 105 cells/ml (THP-1) or 5 × 105 cells/ml (HBE) and incubated for either 3 days (THP-1) or 48 h (HBE). Cells were then incubated at least in triplicate for 6 hours (to measure TNF-α production) or 8 hours (to measure IL-8 production) either in the presence of medium alone or with Escherichia coli O111:B4 LPS (Sigma Chemical Co., St Louis, Mo.), cationic peptides, or a combination of LPS and peptide (concentrations are stated in the text) in medium. Supernatants were collected and stored at −20°C until use.
Chemotaxis assay.
Chemotaxis of the monocyte-like cell line THP-1 was assayed with a 96-well disposable chemotaxis system with 5-μm polycarbonate filters (Chemotx; Neuroprobe, Gaithersburg, MD). Chemotactic factors were diluted in RPMI medium supplemented with 1% bovine serum albumin (chemotaxis medium) and added to the bottom chamber of the chemotaxis plate. A 106-cell/ml solution of THP-1 cells was made in chemotaxis medium, and 50 μl was added to the top of the chamber. The plate was incubated at 37°C in 5% CO2 for 3 hours. In order to quantify the number of cells that migrated through the filter, a standard curve of cell number represented by color change was created using a colorimetric assay of cell viability based on cleavage of tetrazolium salt WST-1 (Roche Diagnostics). WST-1 (10%, vol/vol) was added, and the plate was incubated at 37°C in 5% CO2 until color development was complete. The plate was then read at 570 nm with a PowerWave 340 ELISA plate reader. The number of cells that had migrated through the membrane was expressed as a percentage of the total number of cells added (chemotaxis index).
Statistical analysis.
Student's t test was performed to determine statistical significance. Values are expressed as means ± standard errors. Significance was determined as a P value of <0.05.
RESULTS
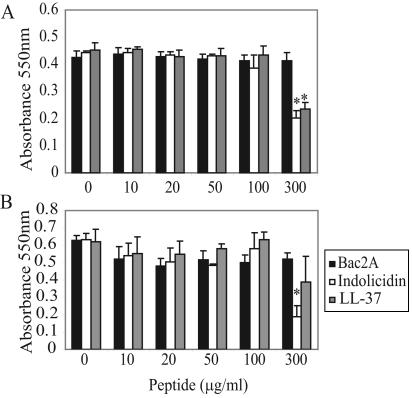
Indolicidin, Bac2A, and LL-37 are not cytotoxic at concentrations used in this study.
Cytotoxicity was assessed using the MTT assay. None of the peptides caused significant cytotoxicity in either the HBE or THP-1 cell line at concentrations of ≤100 μg/ml (Fig. 1). In contrast, the cytotoxic peptide CP29 caused significant cell death at a concentration of 50 μg/ml (data not shown).
FIG. 1.
LL-37, Bac2A, and indolicidin are not cytotoxic to THP-1 cells (A) or HBE cells (B) at concentrations ≤100 μg/ml. At 300 μg/ml, significant cytotoxicity was observed in indolicidin-treated HBE cells and in indolicidin- and LL-37-treated THP-1 cells. Mean values ± standard errors of the mean of three independent experiments are represented. A Student's t test was performed, and asterisks denote significance at a P value of <0.005 compared to cells treated with a vehicle control only.
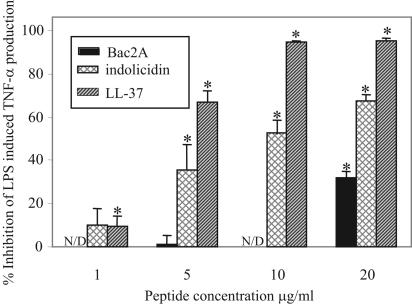
Indolicidin, but not Bac2A, displays potent antiendotoxic properties.
Certain cationic peptides have the ability to block the production of cytokines produced in response to LPS by either directly up-regulating inhibitory pathways in cells (26) or interfering with the ability of LPS to bind LPS-binding protein (27). THP-1 cells were stimulated with E. coli LPS, and the amount of TNF-α in the supernatant was assayed by ELISA. The cells produced between 2,600 and 3,900 ng/ml of TNF-α. The ability of the short peptides indolicidin and Bac2A to inhibit LPS-induced TNF-α production in the THP-1 cell line was investigated at low concentrations of host defense peptides such as would be found at mucosal surfaces. The addition of indolicidin at the same time as LPS suppressed the LPS-induced production of TNF-α in a dose-dependent manner (Fig. 2) in the differentiated macrophage-like THP-1 cell line and was statistically significant at ≥5 μg/ml (P < 0.001). This inhibition was not as potent at low concentrations as was observed for LL-37 (Fig. 2), a human cathelicidin previously shown to have antiendotoxin ability (27); however, at concentrations of 50 μg/ml, over 80% inhibition was observed (data not shown). In contrast, Bac2A caused no inhibition over the concentration range of 1 to 10 μg/ml and demonstrated only moderate antiendotoxin activity (approximately 30%) at 20 μg/ml. Interestingly, as shown previously with LL-37 and the insect-derived peptide CEMA (10, 24), the inhibition of cytokine production was observed even when indolicidin was added to the THP-1 cells up to 1 hour after LPS (data not shown). This indicates that the antiendotoxin properties of indolicidin may be due to an interaction of the peptide with cells in addition to direct neutralization of LPS.
FIG. 2.
Dose-dependent inhibition of LPS-induced TNF-α by addition of peptides. Indolicidin and LL-37 significantly reduced the amount of TNF-α produced by differentiated THP-1 cells upon stimulation with 100 ng/ml of E. coli O111:B4 LPS. Bac2A did not reduce the amount of TNF-α at the concentrations tested (N/D, no detectable decrease in TNF-α). Mean values ± standard error of the mean of one representative experiment of at least three are represented. A Student's t test was performed, and asterisks denote significance at a P value of <0.001 compared to controls treated with LPS only.
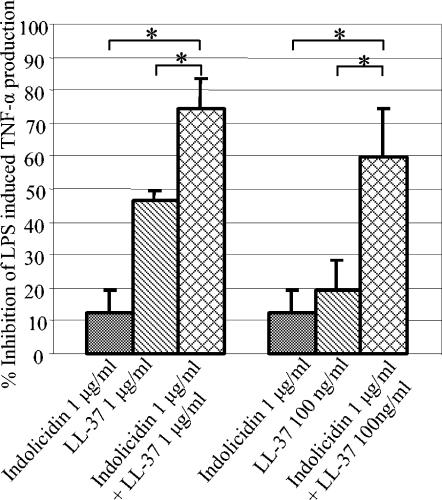
The antiendotoxin activity of indolicidin and LL-37 in combination is greater than that of the additive effect.
It has been well established that certain cationic peptides used in combination can demonstrate synergistic antimicrobial activity (39). To determine whether this phenomenon was applicable to the nonantimicrobial activities of cationic peptides, we examined the ability of LL-37 to work synergistically with indolicidin to inhibit LPS-induced TNF-α production. The antiendotoxin activity of indolicidin and LL-37 in combination was significantly greater than that of either peptide alone and greater than that of the additive effect of these two peptides (Fig. 3) (P < 0.05). In contrast, the addition of human cationic peptides such as human neutrophil peptide 1 or human beta defensins 1 and 2 with either indolicidin or LL-37 produced an additive rather than a synergistic effect (data not shown).
FIG. 3.
The addition of indolicidin and LL-37 in combination produces a greater-than-additive inhibition of LPS-induced TNF-α. Indolicidin and LL-37 significantly reduced the amount of TNF-α produced by differentiated THP-1 cells upon stimulation with 100 ng/ml of E. coli O111:B4 LPS. The combination of very small amounts of indolicidin (1 μg/ml) or LL-37 (1 μg/ml or 100 ng/ml) resulted in a greater reduction of LPS-induced TNF-α than would be predicted to occur if this effect was strictly additive. Mean values of the averages of three independent experiments ± the standard error of the mean are shown. A one-tailed Student's t test was performed, and the results significant at a P value of <0.05 are marked with an asterisk.
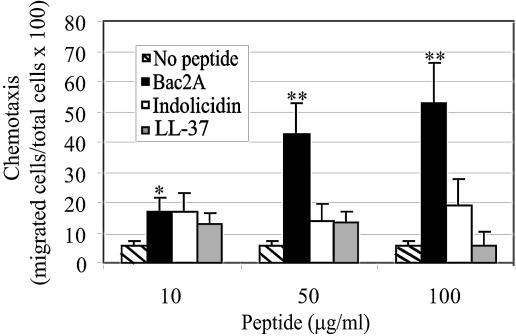
Bac2A is a more potent chemotactic agent than indolicidin.
It has been shown previously that LL-37 is chemotactic for various cells types, including monocytes, mast cells, and T cells (5, 19). Little is known about the chemoattractant properties of other cathelicidins, although a relative of bactenecin, Bac7, has been shown to chemoattract monocytes (35). In this experiment, the abilities of indolicidin, Bac2A, and LL-37 to induce chemotaxis of undifferentiated THP-1 cells were investigated. The addition of Bac2A to the bottom chamber of the Neuroprobe Chemotx filter induced the migration of THP-1 cells in a statistically significant (P < 0.05 at 10 μg/ml Bac2A and P < 0.001 at ≥50 μg/ml Bac2A) and dose-dependent manner (Fig. 4). Indolicidin did not induce a statistically significant increase in chemotaxis, except at a concentration of 300 μg/ml, at which concentration it demonstrated some cytotoxicity. Interestingly, LL-37, which has previously been shown to be chemotactic for blood-derived monocytes (5), was not chemotactic for THP-1 cells.
FIG. 4.
Bac2A is a more potent chemoattractant for undifferentiated THP-1 cells than indolicidin. Indolicidin, Bac2A, LL-37, or water (vehicle control) was added to the bottom well of a chemotaxis chamber at the concentrations shown. Undifferentiated THP-1 cells were added to the top well, and after 3 hours, chemotaxis was assessed. Chemotaxis was measured by calculating the total number of cells that migrated as a percentage of the total number of cells added. Mean values of the averages of three independent experiments ± the standard error of the mean are shown. A two-tailed t test was performed, and the results significant at a P value of <0.05 are marked with an asterisk, and those significant at a P value of <0.001 are marked by a double asterisk.
Indolicidin, but not Bac2A, can induce IL-8 expression in 16HBE4o− cells.
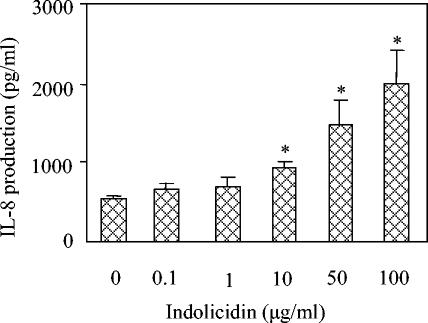
It has been shown previously that LL-37 induces the release of the chemokines IL-8 and/or MCP-3 in both the murine lung and various cell lines (24). Therefore, we investigated the possibility that indolicidin and/or Bac2A might have similar properties. In differentiated THP-1 cells, indolicidin inhibited the LPS-induced production of the cytokine TNF-α but did not itself induce TNF-α or IL-8 (data not shown). However, in the human bronchial cell line 16HBE4o−, indolicidin concentrations of ≥10 μg/ml induced significant production of the chemokine IL-8 (P < 0.05) in a dose-dependent manner (Fig. 5) but did not induce TNF-α (data not shown). Bac2A did not induce any cytokine or chemokine production, even at 50 μg/ml (data not shown).
FIG. 5.
Indolicidin induces IL-8 production in a dose-dependent manner in the human bronchial epithelial cell line 16HBE4o−. Cells were grown to confluency and subsequently incubated with indolicidin for 8 hours. The presence of IL-8 in the supernatant was detected by ELISA. Mean values ± standard errors of the means of one representative experiment of at least three are represented (n = 4 replicates for each condition). A two-tailed t test was performed, and results significant at a P value of <0.05 are marked with an asterisk.
DISCUSSION
In this study, the bovine neutrophil-derived peptides indolicidin and Bac2A were demonstrated to have diverse and complementary immunomodulatory functions, as summarized in Table 1, in addition to their established antimicrobial activities. Indolicidin inhibited the LPS-induced proinflammatory cytokine responses in a macrophage-like cell line and induced chemokine production in a dose-dependent manner in a bronchial epithelial cell line. Conversely, Bac2A had very weak antiendotoxic or chemokine-inducing properties but acted directly to induce chemotaxis of macrophage-like THP-1 cells.
TABLE 1.
Comparison of the activities of indolicidin, Bac2A, and LL-37
| Property | Minimal concn (μg/ml) demonstrating the given property
|
Reference(s) or source | ||
|---|---|---|---|---|
| Indolicidin | Bac2A | LL-37 | ||
| MIC | ||||
| Gram positive (e.g., Staphylococcus aureus) | 8 | 4 | 64 | 34, 37 |
| Gram negative (e.g., Pseudomonas aeruginosa) | 64 | 8 | 64 | 37, 39 |
| Antiendotoxin activity | 5 | 20 | 1 | Fig. 2 |
| IL-8 production | 10 | ND (>100)a | 10 | 2, 16, Fig. 5 |
| Chemotaxis | ND (>100)b | 10 | ND (>300)c | Fig. 4 |
ND, not detectable at concentrations ≤100 μg/ml.
ND, chemotaxis was observed at 300 μg/ml (data not shown).
ND, not detectable at concentrations ≤300 μg/ml.
Cationic peptides have traditionally been studied as antimicrobial agents. In bovine neutrophils, multiple classes of peptides have been found, including the β-sheet defensins (30), the α-helical peptide BMAP, and the short (12 to 13 amino acids) cationic peptides bactenecin (8) and indolicidin (29). It seems reasonable to assume that these peptides have evolved to work in combination as opposed to independently and that each peptide has distinct (but possibly overlapping) functions. Indeed, β-defensins have different antimicrobial activity spectra (31), while certain combinations of peptides have been demonstrated to be synergistic in their antimicrobial activities (39). However, direct killing of microbes may not always be the primary role that these agents perform in the innate immune response, as the physiological concentrations of peptides and of antagonistic monovalent and divalent cations are often difficult to rationalize with a primary antimicrobial role in vivo (1, 11) Alternatively, their possible primary action is modulation of immune mechanisms, as these functions occur at physiological salt and peptide concentrations (1, 24). However, the degree to which these functions contrast and/or complement each other in different host defense peptides is largely unknown.
Individual peptides have been demonstrated to have particular activities in modulating aspects of innate immunity, although a thorough evaluation of the immunomodulatory effects of most cationic peptides has not been made. To test the possibility that different peptides may have diverse immunomodulatory functions on human cells and thus have alternatively directed potential as immune-boosting drugs, we chose to evaluate the properties of two of the smallest related cationic peptides. It was interesting that Bac2A, despite having a stronger binding affinity than indolicidin for LPS (27), was unable to block LPS-induced TNF-α production at equivalent concentrations. This was consistent with our previous results indicating that the ability of peptides to block LPS-induced responses does not rely entirely on their ability to bind to LPS (28). Indeed, gene array studies on the RAW 264.7 mouse macrophage cell line demonstrated that peptides can block the expression of only a subset of the genes induced by LPS, while themselves inducing the expression of a unique subset of genes (26). This is consistent with the interpretation that the addition of peptides does not merely prevent LPS from binding to the macrophages but alters LPS-induced gene expression in a more directed fashion. Indeed, we showed that indolicidin can block cytokine production when added up to an hour after the addition of LPS to cells (data not shown) as previously demonstrated for LL-37 and the insect peptide CEMA. In this time period, we assume that LPS would already bind to LPS receptors on macrophages and initiate signaling (12). The ability of peptides to selectively block proinflammatory cytokine gene expression implies that although their ability to bind to LPS and disrupt LPS binding to LPS-binding protein (27) may be a component of their antiendotoxin ability, it is not the sole method by which they block proinflammatory responses. Indeed, among the genes that are up-regulated directly by peptides are antiinflammatory cytokines such as IL-10 (24).
The ability of peptides to block the excess cytokine production that is a hallmark of sepsis has led to their consideration as antisepsis agents (3, 13, 17). However, toxicity due to the relatively high concentrations of peptide required is a major concern. In this study, we demonstrate that low concentrations of multiple peptides may be more effective in blocking LPS-induced cytokine responses than higher concentrations of a single peptide and that exogenous addition of peptides might lead to synergy with the host's own naturally occurring peptides.
It has been postulated that antimicrobial peptides have evolved from the deletion products of CXC chemokines (15), and, consistent with this, certain peptides have been shown to be chemotactic for distinct subsets of leukocytes. For example, LL-37 has been shown to be chemotactic for human blood-derived monocytes and T cells (5) through formyl peptide receptor-like 1 (FPRL1) at concentrations of approximately 50 μg/ml, whereas it is chemotactic towards mast cells through two different receptors between 5 and 10 μg/ml (18). Thus, chemotaxis occurs at concentrations that are generally higher than those required for antiendotoxin activity. Although there is a paucity of data indicating the exact concentrations of host defense peptides in vivo, it is known that the cathelicidins are found at extremely high concentrations in the granules of neutrophils, and it seems reasonable that chemotaxis in vivo might occur in response to somewhat higher concentrations of peptides, such as would occur at sites of neutrophil degranulation. Interestingly, in our study, LL-37 was not chemotactic for the THP-1 monocyte-like cell line (Fig. 4) even though this cell line has been shown to express FPRL-1 (21), and we found that these cells express FPRL-1 at the reverse transcriptase PCR level (unpublished results). Conversely, Bac2A, but not indolicidin, was shown to induce chemotaxis of the cells at concentrations of ≥10 μg/ml (Fig. 3). Future experiments will elucidate the chemotactic receptor for Bac2A.
These data indicate that short peptides such as indolicidin and Bac2A possess contrasting immunomodulatory properties which appear to be comparable to certain activities of the known immunomodulator LL-37. Interestingly, these bovine-derived peptides function on human cell lines, indicating that host defense peptides may demonstrate conserved function across species. The differences in their immunomodulatory properties may reflect physiologically complementary functions in vivo and/or be a consequence of the modifications made to the naturally occurring bactenecin to produce the derivative Bac2A. Regardless of this, these contrasting activities make these peptides valuable in the design of alternatively directed therapeutic agents and as tools in dissecting the variations in the mechanisms that underpin these diverse activities. In addition, their smaller size compared to LL-37 makes them potentially exciting prototypes for development as novel immunomodulatory drugs, especially since their collective ability to enhance chemokine production, induce chemotaxis, and block endotoxin responses resembles the properties of LL-37. Future work will involve optimizing these peptides and better characterizing their immunomodulatory properties to further illuminate their potential as novel therapeutic agents.
Acknowledgments
This work was supported by grants from the Canadian Cystic Fibrosis Foundation and the Applied Food and Material Network of Centres of Excellence. R.E.W.H. holds a Canada Research Chair, D.M.E.B. is supported by a CIHR studentship, D.J.D. was funded by a Wellcome Trust UK International Prize Traveling Research Fellowship (060168), and M.G.S. was funded by a CIHR studentship.
REFERENCES
- 1.Bowdish, D. M. E., D. J. Davidson, and R. E. W. Hancock. 2005. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 6:35-51. [DOI] [PubMed] [Google Scholar]
- 2.Bowdish, D. M. E., D. J. Davidson, D. P. Speert, and R. E. W. Hancock. 2004. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 172:3758-3765. [DOI] [PubMed] [Google Scholar]
- 3.Ciornei, C. D., A. Egesten, M. Engstrom, K. Tornebrandt, and M. Bodelsson. 2002. Bactericidal/permeability-increasing protein inhibits endotoxin-induced vascular nitric oxide synthesis. Acta Anaesthesiol. Scand. 46:1111-1118. [DOI] [PubMed] [Google Scholar]
- 4.Cozens, A. L., M. J. Yezzi, K. Kunzelmann, T. Ohrui, L. Chin, K. Eng, W. E. Finkbeiner, J. H. Widdicombe, and D. C. Gruenert. 1994. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 10:38-47. [DOI] [PubMed] [Google Scholar]
- 5.De, Y., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond, G., J. P. Russell, and C. L. Bevins. 1996. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc. Natl. Acad. Sci. USA 93:5156-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falla, T. J., D. N. Karunaratne, and R. E. W. Hancock. 1996. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 271:19298-19303. [DOI] [PubMed] [Google Scholar]
- 8.Gennaro, R., L. Dolzani, and D. Romeo. 1983. Potency of bactericidal proteins purified from the large granules of bovine neutrophils. Infect. Immun. 40:684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gennaro, R., M. Zanetti, M. Benincasa, E. Podda, and M. Miani. 2002. Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr. Pharm. Des. 8:763-778. [DOI] [PubMed] [Google Scholar]
- 10.Gough, M., R. E. W. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, R. E. W., and D. Devine. 2004. “Antimicrobial” or “host defence” peptides?, p. 1-4. In D. Devine and R. E. W. Hancock (ed.), Mammalian host defence peptides. Cambridge University Press, New York, N.Y.
- 12.Hsu, Y. W., K. H. Chi, W. C. Huang, and W. W. Lin. 2001. Ceramide inhibits lipopolysaccharide-mediated nitric oxide synthase and cyclooxygenase-2 induction in macrophages: effects on protein kinases and transcription factors. J. Immunol. 166:5388-5397. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, J., P. Zhu, Z. Wang, Y. He, D. Liu, K. Tian, and Y. Diao. 1998. Protective effect of bactericidal/permeability-increasing protein in mice with E. coli sepsis. Chin. J. Traumatol. 1:21-24. [PubMed] [Google Scholar]
- 14.Kim, S. T., H. E. Cha, D. Y. Kim, G. C. Han, Y. S. Chung, Y. J. Lee, Y. J. Hwang, and H. M. Lee. 2003. Antimicrobial peptide LL-37 is upregulated in chronic nasal inflammatory disease. Acta Otolaryngol. 123:81-85. [DOI] [PubMed] [Google Scholar]
- 15.Krijgsveld, J., S. A. Zaat, J. Meeldijk, P. A. van Veelen, G. Fang, B. Poolman, E. Brandt, J. E. Ehlert, A. J. Kuijpers, G. H. Engbers, J. Feijen, and J. Dankert. 2000. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 275:20374-20381. [DOI] [PubMed] [Google Scholar]
- 16.Lau, Y. E., A. Rozek, M. G. Scott, D. L. Goosney, D. J. Davidson, and R. E. W. Hancock. 2005. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect. Immun. 73:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin, M., P. A. Quint, B. Goldstein, P. Barton, J. S. Bradley, S. D. Shemie, T. Yeh, S. S. Kim, D. P. Cafaro, P. J. Scannon, B. P. Giroir, et al. 2000. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet 356:961-967. [DOI] [PubMed] [Google Scholar]
- 18.Niyonsaba, F., K. Iwabuchi, A. Someya, M. Hirata, H. Matsuda, H. Ogawa, and I. Nagaoka. 2002. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyonsaba, F., A. Someya, M. Hirata, H. Ogawa, and I. Nagaoka. 2001. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur. J. Immunol. 31:1066-1075. [DOI] [PubMed] [Google Scholar]
- 20.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 21.Resnati, M., I. Pallavicini, J. M. Wang, J. Oppenheim, C. N. Serhan, M. Romano, and F. Blasi. 2002. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad. Sci. USA 99:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romeo, D., B. Skerlavaj, M. Bolognesi, and R. Gennaro. 1988. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 263:9573-9575. [PubMed] [Google Scholar]
- 23.Rozek, A., C. L. Friedrich, and R. E. W. Hancock. 2000. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 39:15765-15774. [PubMed] [Google Scholar]
- 24.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. W. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 25.Scott, M. G., and R. E. W. Hancock. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407-431. [PubMed] [Google Scholar]
- 26.Scott, M. G., C. M. Rosenberger, M. R. Gold, B. B. Finlay, and R. E. W. Hancock. 2000. An alpha-helical cationic antimicrobial peptide selectively modulates macrophage responses to lipopolysaccharide and directly alters macrophage gene expression. J. Immunol. 165:3358-3365. [DOI] [PubMed] [Google Scholar]
- 27.Scott, M. G., A. C. Vreugdenhil, W. A. Buurman, R. E. W. Hancock, and M. R. Gold. 2000. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 164:549-553. [DOI] [PubMed] [Google Scholar]
- 28.Scott, M. G., H. Yan, and R. E. Hancock. 1999. Biological properties of structurally related α-helical cationic antimicrobial peptides. Infect. Immun. 67:2005-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selsted, M. E., M. J. Novotny, W. L. Morris, Y. Q. Tang, W. Smith, and J. S. Cullor. 1992. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 267:4292-4295. [PubMed] [Google Scholar]
- 30.Selsted, M. E., Y. Q. Tang, W. L. Morris, P. A. McGuire, M. J. Novotny, W. Smith, A. H. Henschen, and J. S. Cullor. 1993. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 268:6641-6648. [PubMed] [Google Scholar]
- 31.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes, R. W., and D. Doxsee. 1999. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell. Immunol. 197:1-9. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 34.Turner, J., Y. Cho, N. N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbanac, D., M. Zanetti, and D. Romeo. 1993. Chemotactic and protease-inhibiting activities of antibiotic peptide precursors. FEBS Lett. 317:255-258. [DOI] [PubMed] [Google Scholar]
- 36.Woo, J. S., J. Y. Jeong, Y. J. Hwang, S. W. Chae, S. J. Hwang, and H. M. Lee. 2003. Expression of cathelicidin in human salivary glands. Arch. Otolaryngol. Head Neck Surg. 129:211-214. [DOI] [PubMed] [Google Scholar]
- 37.Wu, M., and R. E. W. Hancock. 1999. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrob. Agents Chemother. 43:1274-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, M., E. Maier, R. Benz, and R. E. W. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 39.Yan, H., and R. E. W. Hancock. 2001. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, G., H. Wu, C. R. Ross, J. E. Minton, and F. Blecha. 2000. Cloning of porcine NRAMP1 and its induction by lipopolysaccharide, tumor necrosis factor alpha, and interleukin-1β: role of CD14 and mitogen-activated protein kinases. Infect. Immun. 68:1086-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]