Abstract
Sleep apnea (SA) is associated with risk of cardiovascular disease, cognitive decline, and accidents due to sleepiness, yet the majority (over 80%) of patients remain undiagnosed. Inertial measurement units (IMUs) are built into modern wearable devices and are capable of long-term continuous measurement with low power consumption. We examined if SA can be detected by an IMU embedded in a wristwatch device. In 122 adults who underwent polysomnography (PSG) examinations, triaxial acceleration and triaxial gyro signals from the IMU were recorded during the PSG. Subjects were divided into a training group and a test groups (both n = 61). In the training group, an algorithm was developed to extract signals in the respiratory frequency band (0.13–0.70 Hz) and detect respiratory events as transient (10–90 s) decreases in amplitude. The respiratory event frequency estimated by the algorithm correlated with the apnea–hypopnea index (AHI) of the PSG with r = 0.84 in the test group. With the cutoff values determined in the training group, moderate-to-severe SA (AHI ≥ 15) was identified with 85% accuracy and severe SA (AHI ≥ 30) with 89% accuracy in the test group. SA can be quantitatively detected by the IMU embedded in wristwatch wearable devices in adults with suspected SA.
Subject terms: Data processing, Respiration, Diagnostic markers
Introduction
Sleep apnea (SA) is characterized by repeated episodes of apnea and hypopnea due to upper airway collapse and obstruction or cessation of central respiratory drive during sleep associated with subsequent arousal from sleep. SA is a common health problem affecting 10–30% of adults1,2, but the consequences are serious3. For instance, poor sleep quality due to SA is associated with daytime sleepiness that reduces work performance and cognitive ability and increases the risk of traffic accidents4–6. Recurrent nocturnal hypoxia, hypercapnia, and sustained sympathetic activation caused by SA7 are associated with increased risks of cognitive dysfunction8, systemic hypertension9, atrial fibrillation10,11, stroke12, and nocturnal sudden cardiac death13. Polysomnography (PSG) is the gold-standard method for the detection and diagnosis of SA in adults, but there are practical limitations due to the number of PSG testing facilities and testing costs. To overcome this limitation, a number of home portable devices have been proposed for out-of-clinic sleep testing (OCST)14 with acceptable agreement with PSG results15,16. However, patients with SA, even if they have symptoms such as insomnia or daytime sleepiness, are unlikely to be examined with those SA testing devices, unless they suspect that their symptoms may be caused by SA. In fact, the majority (over 80%) of SA patients still remains undiagnosed6 and potential patients with SA are considered a major burden of the healthcare system3. There is a need to develop screening methods for SA that have the opportunity to be accessed by more people, including those who may be unaware that they have SA or unaware of the need for SA testing.
Inertial measurement units (IMUs) are embedded in most modern wearable devices and enable continuous measurement of triaxial acceleration and triaxial gyro signals for long period of time with low power consumption. If SA can be detected with IMUs, especially those built into widespread wristwatch-type wearable devices, it is expected that the probability of detecting potential patients will increase. In a previous study, we reported that SA can be detected quantitatively by the analysis of pulse rate data from a wristwatch device’s photoplethysmography (PPG) sensor during sleep17, but the PPG sensor requires frequent (usually once a day) battery charging because it consumes more power than IMUs. Additionally, the detection of SA by pulse rate relies on the faithful coupling of SA events and heart rate (cyclic variation of heart rate)18,19, which could be impaired by cardiac and neural comorbidity 20. In this study, we examined the possibility of detecting SA using the output signal of an IMU embedded in a wristwatch wearable device. This is the first study to show that SA can be detected by an IMU worn on the wrist.
Methods
Ethics approval and consent to participate
All procedures were performed in accordance with the Regulations Concerning the Conduct of Life Science and Medical Research Involving Human Subjects at Tohoku University, Japan, the Ethical Guidelines for Medical Research Involving Human Subjects issued by the Japanese Ministry of Health, Labor and Welfare, and the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the Research Ethics Committee of the Center for Data-driven Science and Artificial Intelligence, Tohoku University, Japan (No. 2022-4). All subjects participated in this study gave their written informed consent.
Subjects
The subjects were patients who underwent overnight PSG from November 2022 to March 2023 at Takaoka Clinic (Nagoya, Japan) for the diagnosis of SA or for the evaluation of SA treatment efficacy. The inclusion criterion was adults of age ≥ 20 years. Subjects were excluded if they had acute illness, infectious disease, or chronic disease exacerbation requiring hospitalization within the last 3 months, or were pregnant or breastfeeding.
Protocol
Subjects visited the sleep clinic in the evening and slept in a PSG testing chamber equipped with an Embla N7000 PSG amplifier (Natus Neurology Incorporated, Middleton, Wisconsin, USA). During PSG testing, a wristwatch device (mSafety, Sony Network Communications Europe, Malmö, Sweden) was worn on the left wrist, and triaxial acceleration and triaxial gyro signals from a built-in IMU (BMI270, Bosch Sensortec GmbH, Reutlingen, Germany) and pulse wave signals from a built-in PPG sensor were recorded simultaneously with the PSG (the pulse wave signals were not used in this study).
Subjects were randomly allocated into a training group and a test group. Using the data from the training group, we developed algorithms to identify SA episodes, constructed regression models to estimate SA severity, and determined the optimal cutoff values for classifying the severity. Using the data from the test group, we evaluated the prediction and classification performance of the algorithms. The measurements were conducted under identical condition between the training and test groups.
Measurements
The PSG was recorded overnight with the standard PSG montages consisting of F4-M1, F3-M2, C4-M1, C3-M2, O2-M1, and O1-M2 electroencephalograms, left and right electrooculograms, a submental electromyogram, a nasal pressure cannula, oronasal airflows, left and right tibial electromyograms, thoracoabdominal inductance plethysmograms, pulse oxy-metric arterial blood oxygen saturation (SpO2), a neck microphone, body position sensors, and a modified lead II ECG.
The PSG recoding was analyzed offline with a sleep diagnostic software (Remlogic version 3.4.1, Natus Medial Incorporated, Middleton, Wisconsin, USA) and the results of automated analysis were reviewed and edited by expert sleep technicians (Certified Sleep Medicine Examiner by the Japan Sleep Society). Sleep stages and respiratory events were scored according to the American Association of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events, Version 2.521. The average hourly frequencies of apneic episodes, hypopneic episodes, and the combination were defined as apneic index, hypopneic index, and apnea–hypopnea index (AHI), respectively. The average hourly frequencies of apneic episodes were also measured by the types (obstructive, central, and mixed). AHI was calculated both using the total recording time (TRT) as the denominator (AHITRT) and total sleep time (TST) as the denominator (AHITST). The AHITRT was used as the reference for the development of the algorithm for detection of respiratory events by IMU signals. The AHITST was used to determine the severity class of SA in the subjects: subjects with an AHITST between 15 and 30 as having moderate SA and those with AHITST ≥ 30 as having severe SA.
The acceleration signal of each axis was recorded at a sampling frequency of 32 Hz and a resolution of 0.061 mG per least significant bit (± 2.0 G at 16 bit) and the gyro signal of each axis was at a sampling frequency of 32 Hz and a resolution of 0.0076 degree per second (dps) per least significant bit (± 250 dps at 16 bit). Acceleration, gyro, and PPG signals were transferred from the wristwatch device to a secure cloud storage via the device's built-in long-term-evolution-for-machine (LTE-M) communication function for constant network connectivity.
Data analysis
The acceleration signals and gyro signals were analyzed in the same way but separately. For both signals, the entire length (equivalent to the TRT of PSG) of time series were processed as follows. First, each time series of the three axes (X, Y, and Z) was processed separately with a band-pass filter between 0.13 and 0.70 Hz to extract the respiratory signals. Second, the triaxial respiratory signals were combined into a single scalar that reflects respiratory wrist movement,
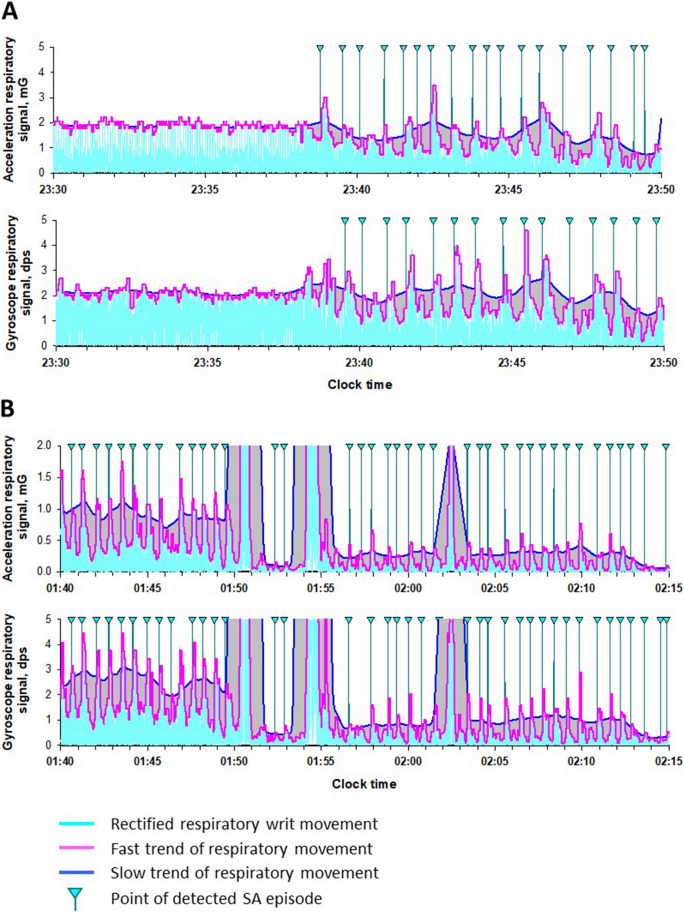
where Rt represents time series of respiratory wrist movement and Xt, Yt , and Zt indicate band-pass filtered X-, Y-, and Z-axis time series, respectively. Third, the time series Rt was band-pass filtered (0.13–0.7 Hz) again to remove the direct-current component and rectified so that the height of upper envelope of the data reflects the amplitude of respiratory wrist movement (Fig. 1). Fourth, considering that the fast trend in the upper envelope reflect the breath-by-breath change in the amplitude of respiratory wrist movement and the slow trend reflects the mean amplitude flattening the SA-induced change in the amplitude, we calculated moving averages of the envelope with averaging window widths of 3 and 30 s for the fast and slow trends, respectively. Fifth, we searched for fast trend drops where the area bounded by the fast and slow trends was greater than a threshold percentage of area under the curve (AUC) of the slow trend, and determined the drop as an SA episode if its length was between 10 and 90 s. Finally, the average number of SA episodes per hour of TRT was calculated as the respiratory event index (REI).
Figure 1.
Detection of sleep apnea–hypopnea by acceleration and gyro signals from inertial measurement unit (IMU). (A) Rectified respiratory wrist movement (cyan) derived from acceleration (upper row) and gyro (lower row) signals during the transition from normal breathing to a train of sleep apnea (SA) in a representative subject with sever SA. (B) Rectified respiratory wrist waveforms (cyan) from acceleration (upper panel) and gyro (lower panel) signals with varying signal intensities during successive episodes of sleep apnea in the same subject. In all panels, blue and magenta lines indicate slow and fast trends, respectively, in the upper envelope of respiratory wrist movement. A drop in the fast trend was determined to be an SA episode if the area bounded by the fast and slow trends was greater than the threshold percentage of area under the slow trend curve and was between 10 and 90 s in the duration. Vertical lines with cyan triangles indicate the points at which an SA episode was detected.
As shown in the first 8 min of Fig. 1A, the fast and slow trends during normal breathing overlap each other and the area bounded by them is small or nonexistence; when SA episode occurs, drop in the fast trend appears and the area bounded by the fast and slow trends increase. The amplitude of respiratory wrist movement could vary with arm and body positions (Fig. 1B). To eliminate the influence of amplitude changes, we evaluated the ratio of the bounded area to the AUC of the slow trend as an index for detecting SA episodes. The threshold for this ratio to detect SA episodes was determined to maximize the multiple correlation coefficient for the AHITRT regression with acceleration REI and gyro REI in the training group.
Statistical analysis
The program package of Statistical Analysis System (SAS institute, Cary, NC, USA) was used for statistical analyses. Differences in quantitative and categorical variables between the training and test groups were evaluated by Wilcoxon rank sum test and χ2 test. The relationship between acceleration/gyro REI and AHITRT was evaluated by linear regression analysis and Pearson's correlation coefficient, and the multivariate predictive power of acceleration and gyro REIs for AHITRT was evaluated by multiple correlation coefficient using the SAS REG procedure. The AHITRT estimated by the multiple regression model was used as the respiratory event score (RES). The discriminant performance of the RES between dichotomized subjects by SA severity (cutoff points, AHITST ≥ 15 and ≥ 30) was evaluated by the AUC of receiver-operating characteristic (ROC) curve. The optimal cutoff values of the RES for the discrimination were determined in the training group and evaluated in the test group with the sensitivity, specificity, accuracy, and positive and negative predictive values (PPV and NPV, respectively). Statistical significance was considered for P < 0.05.
Results
Subjects’ characteristics
We studied 122 subjects (age range, 21 to 73 years, 14 females) who underwent PSG for diagnostics (n = 97, 81%) and evaluation of therapeutic effects (n = 25, 19%). Their median (IQR) body mass index was 26.2 (23.9 to 29.7) kg/m2. The results of PSG showed that their median (IQR) AHITST was 17.5 (8.7 to 34.6), that 67 (55%) of them had a moderate-to-severe SA (AHITST ≥ 15) and 38 (31%) had a severe SA (AHITST ≥ 15), and that the proportion of obstructive, central, and mixed apnea episodes were 87%, 7%, and 6%, respectively.
For this study, the subjects were randomly divided into the training group (n = 61) and the test group (n = 61). There was no significant difference in subjects’ characteristics between the two groups (Table 1).
Table 1.
Subjects’ characteristics in training and test groups.
| Training group (n = 61) | Test group (n = 61) | Pa | |
|---|---|---|---|
| Age, year | 49 (41–55) | 48 (40–53) | 0.4 |
| Female, n (%) | 6 (10%) | 8 (13%) | 0.5 |
| BMI, kg/m2 | 26.0 (23.1–29.4) | 26.7 (24.1–30.1) | 0.3 |
| Purpose of polysomnography | 0.3 | ||
| Diagnosis, n (%) | 52 (85%) | 45 (76%) | |
| CPAP titration, n (%) | 4 (7%) | 8 (14%) | |
| Other, n (%) | 5 (8%) | 6 (10%) | |
| TRT, min | 521.5 (508.5–543.0) | 515.5 (503.5–531.5) | 0.1 |
| Sleep period time, min | 507.0 (491.5–528.5) | 502.5 (485.0–524.0) | 0.2 |
| TST, min | 444.0 (363.5–481.5) | 440.0 (402.5–475.0) | 0.9 |
| Sleep efficiency, % | 821.9 (69.8–92.3) | 83.1 (76.2–91.4) | 0.6 |
| Sleep latency, min | 4.5 (2.5–8.5) | 6.0 (2.5–11.5) | 0.5 |
| Wake after sleep onset, min | 67.0 (34.0–116.5) | 52.5 (33.5–99.0) | 0.4 |
| Sleep stage | |||
| N1, % | 24 (17–39) | 30 (19–39) | 0.3 |
| N2, % | 54 (45–61) | 52 (42–61) | 0.5 |
| N3, % | 0 (0–0) | 0 (0–0) | 0.3 |
| REM, % | 19 (14–23) | 18 (15–21) | 0.2 |
| AHITRT | 15.1 (6.6–24.6) | 14.3 (7.8–30.5) | 0.5 |
| AHITST | 17.3 (8.7–34.6) | 18.1 (9.1–34.0) | 0.7 |
| AITST | 3.6 (1.0–13.6) | 4.3 (0.9–15.9) | 0.6 |
| HITST | 10.4 (6.1–16.7) | 12.2 (6.5–17.9) | 0.5 |
| OAITST | 2.3 (0.2–12.9) | 3.0 (0.4–15.7) | 0.4 |
| CAITST | 0.3 (0.1–0.8) | 0.3 (0.0–0.7) | 0.9 |
| MAITST | 0 (0–0.3) | 0.1 (0.0–0.4) | 0.2 |
| AHITST ≥ 15 | 33 (54%) | 34 (56%) | 0.8 |
| AHITST ≥ 30 | 19 (31%) | 19 (31%) | 1.0 |
Data are median (IQR) or frequency (%).
AHITRT apnea–hypopnea index calculated using total recording time as the denominator, AHITST apnea–hypopnea index calculated using total sleep time as the denominator, AI apnea index, BMI body mass index, CAI central apnea index, CPAP continuous positive airway pressure, HI hypopnea index, OAI obstructive apnea index, MAI mixed apnea index, REM rapid eye movement, TRT total recording time, TST total sleep time.
aSignificance of difference (χ2 test and Wilcoxon rank sum test for categorical and continuous variables, respectively).
Development of algorithm to detect SA episodes using IMU signals in the training group
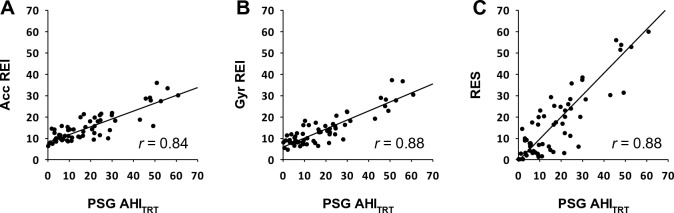
SA detection algorithms for acceleration and gyro signals were developed using data in the training group. The multiple regression of AHITRT by acceleration and gyro REIs indicated that the greatest squared multiple correlation coefficient (R2 = 0.78) was obtained when a threshold of 35% was used to identify drop of the fast trend as an SA episode (Table 2). Therefore, in the following analysis, we decided to use 35% threshold for SA detection. The REI calculated from the acceleration and gyro signals by the SA detection algorithm correlated with the AHITRT in the training group with correlation coefficients of 0.84 and 0.88, respectively (Fig. 2A, B).
Table 2.
Correlations between REIs and RES and AHITRT obtained at different thresholds of SA detection in the training group.
| Thresholda | Acceleration | Gyro | Multiple regression R2d | ||
|---|---|---|---|---|---|
| REIb | rc | REIb | rc | ||
| 10% | 49.0 ± 6.8 | −0.12 | 45.9 ± 6.5 | 0.14 | 0.27 |
| 20% | 34.7 ± 5.7 | 0.5 | 29.0 ± 7.5 | 0.74 | 0.58 |
| 25% | 26.4 ± 6.1 | 0.73 | 22.4 ± 8.0 | 0.83 | 0.69 |
| 30% | 19.6 ± 6.7 | 0.81 | 17.4 ± 7.9 | 0.87 | 0.77 |
| 35% | 14.9 ± 6.8 | 0.84 | 13.7 ± 7.4 | 0.88 | 0.78 |
| 40% | 11.8 ± 6.6 | 0.84 | 10.9 ± 6.8 | 0.87 | 0.76 |
| 45% | 9.6 ± 6.2 | 0.83 | 9.0 ± 6.1 | 0.86 | 0.74 |
| 50% | 8.1 ± 5.8 | 0.83 | 7.6 ± 5.6 | 0.85 | 0.74 |
| 60% | 6.3 ± 5.1 | 0.83 | 6.0 ± 4.9 | 0.83 | 0.71 |
aThreshold for detecting respiratory wrist movement reduction based on the ratio of the area bounded by the fast and slow trends to the area under the slow trend (see Fig. 1).
bRespiratory event index: number of detected events of respiratory wrist movement reduction by acceleration and gyro signals per hour of TRT.
cCorrelation coefficient between REI and AHITRT.
dSquared multiple correlation coefficient for AHITRT regression with acceleration REI and gyro REI. A threshold of 35% resulted in the best estimate of AHITRT.
Figure 2.
Relationships between apnea–hypopnea index (AHITRT) of polysomnography (PSG) and respiration event index (REI) and respiration event score (RES) obtained from IMU signals in the training group. (A) PSG AHITRT and REI obtained from acceleration signal (Acc); (B) PSG AHITRT and REI obtained from gyro signal (Gyr); (C) PSG AHITRT and RES, an estimate of AHITRT by combining Acc REI and Gyr REI. r = correlation coefficient.
Development of a respiratory event score (RES) to estimate AHITRT in the training group
In the training group, REIs obtained from the acceleration and gyro signals were correlated with each other (r = 0.93) and there was no significant difference between them (median [IQR], 13.7 [9.8, 18.3] for acceleration and 11.7 [8.1, 16.5] for gyro), but which was larger differed from subject to subject. Therefore, we decided to create a model that combines both to calculate RES as an estimate of AHITRT. Figure 2c shows the relationship between the PSG AHITRT and RES obtained from the multivariate regression model. The regression model was.
where REIacc and REIgyr represent the REI obtained from acceleration and gyro signals. ROC analysis of the discrimination performance of RES in identifying subjects with moderate-to-severe SA (AHITST ≥ 15) and those with severe SA (AHITST ≥ 30) showed AUC of 0.894 (95%CI, 0.788 to 0.958) and 0.919 (0.820 to 0.973), respectively. The optimal cutoff values for discriminating the subjects with moderate-to-sever SA was RES ≥ 10 (obtained sensitivity, specificity, and accuracy were 85%, 82%, and 84%, respectively) and for those with severe SA was RES ≥ 20 (obtained sensitivity, specificity, and accuracy were 79%, 83%, and 82%, respectively).
Validation of algorithms and model in the test group
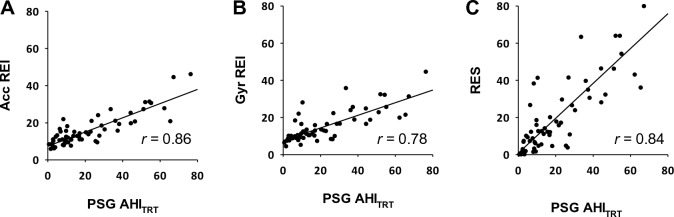
The REIs of the acceleration and gyro signals calculated by the algorithm were closely correlated (r = 0.86 and 0.78, respectively) with AHITRT in the test group as well (Fig. 3A, B). The RES calculated by the regression model also showed a good correlation (r = 0.84) with AHITRT in the test group (Fig. 3C). Additionally, ROC analysis of the discrimination performance of RES in identifying subjects with moderate-to-severe SA (AHITST ≥ 15) and those with severe SA (AHITST ≥ 30) showed AUC of 0.891 (95%CI, 0.785 to 0.956) and 0.921 (0.823 to 0.975), respectively. Tables 3 and 4 show the confusion tables for identification of subjects with different SA severity in the test group using the cutoff values for RES that were determined in the training group. The RES with those cutoff values discriminated moderate-to-severe SA and severe SA with accuracies of 85% and 89%, respectively.
Figure 3.
Relationships of (A) Acc REI, (B) Gyro REI, and (C) RES with AHITRT of PSG in the test group. Acc REI and Gyro REI were calculated from the IMU signals of the test group by the algorithm developed in the training group. The RES was calculated using the regression model developed in the training group. r = correlation coefficient.
Table 3.
Confusion table for identification of moderate to severe SA (AHITST ≥ 15) by RES in the test group.
| AHITST < 15 | AHITST ≥ 15 | ||
|---|---|---|---|
| RES < 10a | 21 | 3 | NPA = 88% |
| RES ≥ 10a | 6 | 31 | PPA = 84% |
| Specificity = 78% | Sensitivity = 91% | Accuracy = 85% |
NPA negative predictive accuracy, PPA positive predictive accuracy.
aCutoff value of 10 was predetermined in the training group.
Table 4.
Confusion table for identification of severe SA (AHITST ≥ 30) by RES in the test group.
| AHITST < 30 | AHITST ≥ 30 | ||
|---|---|---|---|
| RES < 20a | 37 | 2 | NPA = 95% |
| RES ≥ 20a | 5 | 17 | PPA = 77% |
| Specificity = 88% | Sensitivity = 90% | Accuracy = 89% |
aCutoff value of 20 was predetermined in the training group.
Table 5 shows the prevalence of moderate-to-severe SA for multiple levels of RES. If the RES was less than 5, the prevalence of having moderate to severe SA was 17%, but if the RES was 30 or higher, the prevalence was 94%, and if the RES was 40 or higher, the prevalence was 100%. To evaluate the classification performance independent of pretest probability, likelihood ratio was also calculated for each threshold.
Table 5.
Prevalence of moderate to severe SA (AHITST ≥ 15) by multiple thresholds of RES in the test group.
| RES | AHITST < 15 | AHITST ≥ 15 | Positive LR | Prevalence of SA (%) |
|---|---|---|---|---|
| < 10 | 21 | 3 | 0.11 | 13 |
| 10–20 | 4 | 11 | 2.18 | 73 |
| ≥ 20 | 2 | 20 | 7.94 | 91 |
| ≥ 30 | 1 | 16 | 12.7 | 94 |
| ≥ 40 | 0 | 11 | ∞ | 100 |
| Total | 27 | 34 | 56 |
LR likelihood ratio.
Discussion
To the best of our knowledge, this is the first study to demonstrate that SA can be quantitatively detected by an IMU embedded in a wearable device worn on the wrist. In this study, we continuously measured the acceleration and gyro signals from an IMU embedded in a wristwatch device during PSG. Using data from half of the subjects, algorithms were developed to identify SA episodes from respiratory wrist movements detected by the acceleration and gyro signals. We also developed a multivariate model to identify SA severity by estimating AHITRT using SA frequency indices, REIs, provided by the algorithms. In remaining half of the subjects, we tested the accuracies of SA detection and the SA severity classification of the model. We observed that RES, the estimated AHITRT by the model, correlated well with the AHITRT (r = 0.84), and with the pre-defined cutoff values, identified moderate-to-sever SA with 85% accuracy and severe SA with 89% accuracy.
IMU is widely used for sleep medicine. The obvious utility of IMUs is in determining body positioning and detecting body movements during sleep. IMUs worn on the trunk, legs22, and wrists23 have been used for assessing sleep quality and detecting specific changes to neurological disorders such as Parkinson's disease24 and periodic leg movement25. IMUs are also used as sensors to detect seismocardiogram 26, and pulse-synchronized jerks in acceleration detected by a wrist-worn IMU are proposed as an indicator for sleep–wake classification27. However, none of the previous studies have detected SA using IMU signals recorded at the wrist.
In the present study, we found that respiratory movement can be detected by an IMU at the wrist. The resolutions of the acceleration and gyro signals of the IMU were 0.061 mG and 0.0076 dps, respectively, and the observed respiratory wrist movements had amplitudes 10 to 1000 times greater than those. The amplitude of respiratory wrist movement, however, showed frequent and large fluctuations, possibly due to the changes in body posture and the arm position, making the SA detection dependent on a decrease in absolute respiratory amplitude impossible. To overcome this problem, we introduced an algorithm that uses a fast trend reflecting the breath-to-breath changes in respiratory amplitude and a slow trend flattening the amplitude changes due to SA. SA was detected by a local and relative increase in the area bounded by the fast and slow trends above the threshold percentage of the AUC of slow trend. The threshold percentage was determined to maximize the multivariate correlation between the frequency of detected SA episodes and PSG AHITRT.
Three points need to be mentioned about the methods of data analysis of the present study. First, the acceleration and gyro signals were analyzed separately and SA episodes were detected from each signal. The frequencies of detected SA, REIs, were similar and correlated between acceleration and gyro signals. This suggests that respiration causes both linear movements and rotational movements in the wrist. However, which was larger differed from subject to subject. Therefore, we developed a model that combines both to calculate an estimate of AHITRT, i.e., RES. Given the considerable difference in the correlation between REI and AHITRT between the acceleration and gyro signals in the test group (Fig. 3), this method, which does not prioritize one over the other, seems effective. Second, while AHITST is used as a measure of SA severity in clinical PSG testing, we optimized the SA detection algorithm using AHITRT. This was because REI was calculated using TRT as the denominator. On the other hand, AHITST was used for classifying SA severity by PSG. Consequently, the cutoff values of RES were lower than those of AHITST for the SA severity classification. We believe this defined RES cutoff value is practical because it can be used with wearable sensors that do not allow for accurate TST estimation. Finally, a strength of this study is that the development of the SA detection algorithms, the determination of the optimal detection threshold, the creation of the model to estimate AHITRT, and the definition of cutoff values were all done in the training group and validated in the independent test group. This framework ensures the reliability of the study results, at least in similar populations.
The Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea of AASM14 presented the classification performance of six types of Type 3 home SA testing devices in high-risk populations. The reported results indicated sensitivity ranging from 62 to 94%, specificity ranging from 25 to 97%, and accuracy ranging from 65 to 91%. Comparatively, the classification metrics of the present method in the test group were 78%, 91%, and 85%, respectively. Notably, this method measures only two parameters (acceleration and gyro signals), yet it demonstrates favorable performance.
The present findings, demonstrating that SA can be detected by an IMU embedded in a wristwatch device with comparable accuracy to Type 3 devices, open up two possibilities. First, the use of common wristwatch devices for SA screening is to increase the likelihood of detecting SA, especially in those who do not recognize the need for specialized equipment or access to laboratories for SA detection. Given that the majority of SA patients (over 80%) are undiagnosed6 and the serious impact of SA on patients’ quality of life and social and health economics3, increasing SA screening opportunities is an important social need.
Second, the SA detection capability of the IMU may lead to the development of inexpensive, easy-to-use sleep hygiene devices. IMUs consumes less power than PPG sensors, so they require less frequent battery recharging, and there are fewer measurement failures due to sensor detachment from the skin caused by poor fit or intrusion of ambient light, which sometimes occur with PPG. Analysis of pulse data from PPG sensors has also been proposed as a method for quantitative detection of SA17, but this method depends on faithful coupling of SA events to heart rate18,19, which can be compromised by cardiac and neurological comorbidity20.
A limitation of this study is that the algorithm and model were developed in subjects with suspected SA (54% pretest SA probability, odds = 1.27). If the results of this study were applied to the general population with low pretest probability, similar sensitivity and specificity may not be obtained. To predict the results when applied to populations with different prior probabilities, we also calculated positive likelihood ratio for each threshold value of RES (Table 5). Applying the results of the present study to a population with a pretest SA probability of 10%, the expected prevalence of SA is 1%, 20%, 47%, 59%, and ∼100% when RES is < 10, 10–20, ≥ 20, ≥ 30, and ≥ 40, respectively.
Conclusions
Acceleration and gyro signals measured during sleep by an IMU embedded in a wrist-worn wristwatch device can be used to detecting SA episodes and estimating SA severity in adults with suspected SA.
Acknowledgements
Sony Group Corporation, Tokyo, Japan provided funding for this study to Heart Beat Science Lab Inc. and provided the wristwatch devices used in this study.
Author contributions
J.H. and E.Y. conceived the study. J.H. performed the analysis and wrote the main manuscript. M.A. and F.S. contributed to data acquisition and clinical interpretation. E.Y. critically reviewed the manuscript. All authors reviewed the manuscript and approved the submission.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
J.H. is the President and E.Y. is the Chief Technical Officer of Heart Beat Science Lab Inc., which received funding for this study from Sony Group Corporation. J.H. and Sony Group Corporation have filed a patent application for the method of detecting sleep apnea developed in this study. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am. J. Respir. Crit. Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015;7:1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faria A, Allen AH, Fox N, Ayas N, Laher I. The public health burden of obstructive sleep apnea. Sleep Sci. 2021;14:257–265. doi: 10.5935/1984-0063.20200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiomi T, et al. Falling asleep while driving and automobile accidents among patients with obstructive sleep apnea–hypopnea syndrome. Psychiatry Clin. Neurosci. 2002;56:333–334. doi: 10.1046/j.1440-1819.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- 5.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 2009;5:573–581. doi: 10.5664/jcsm.27662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson LM, et al. Sleep disorders and work performance: Findings from the 2008 National Sleep Foundation Sleep in America poll. J. Sleep Res. 2011;20:487–494. doi: 10.1111/j.1365-2869.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 7.Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;78:608–624. doi: 10.1016/j.jacc.2021.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Vanek J, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi: 10.1016/j.sleep.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung P, Anter E. Atrial fibrillation and sleep apnea: Considerations for a dual epidemic. J. Atrial Fibrillation. 2016;8:1283. doi: 10.4022/jafib.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neilan TG, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J. Am. Heart Assoc. 2013;2:e000421. doi: 10.1161/jaha.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunetti V, et al. Sleep and stroke: Opening our eyes to current knowledge of a key relationship. Curr. Neurol. Neurosci. Rep. 2022;22:767–779. doi: 10.1007/s11910-022-01234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gami AS, et al. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur VK, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massie F, Van Pee B, Bergmann J. Correlations between home sleep apnea tests and polysomnography outcomes do not fully reflect the diagnostic accuracy of these tests. J. Clin. Sleep Med. 2022;18:871–876. doi: 10.5664/jcsm.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westenberg JN, et al. Validation of home portable monitoring for the diagnosis of sleep-disordered breathing in adolescents and adults with neuromuscular disorders. J. Clin. Sleep Med. 2021;17:1579–1590. doi: 10.5664/jcsm.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayano J, et al. Quantitative detection of sleep apnea with wearable watch device. PLoS One. 2020;15:e0237279. doi: 10.1371/journal.pone.0237279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984;1:126–131. doi: 10.1016/S0140-6736(84)90062-X. [DOI] [PubMed] [Google Scholar]
- 19.Hayano J, et al. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ. Arrhythm Electrophysiol. 2011;4:64–72. doi: 10.1161/CIRCEP.110.958009. [DOI] [PubMed] [Google Scholar]
- 20.Hayano J, et al. Blunted cyclic variation of heart rate predicts mortality risk in post-myocardial infarction, end-stage renal disease, and chronic heart failure patients. Europace. 2017;19:1392–1400. doi: 10.1093/europace/euw222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry, R. B. etal.TheAASMManualfortheScoringofSleepandAssociationEvents:Rules,TerminologyandTechnicalSpecifications,Version2.5. (American Academy of Sleep Medicine, 2018).
- 22.Buckley N, et al. Can wearable inertial measurement units be used to measure sleep biomechanics? Establishing initial feasibility and validity. Biomimetics (Basel) 2022 doi: 10.3390/biomimetics8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDevitt B, et al. Validity of a novel research-grade physical activity and sleep monitor for continuous remote patient monitoring. Sensors. 2021 doi: 10.3390/s21062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommerauer M, et al. Bound to supine sleep: Parkinson's disease and the impact of nocturnal immobility. Parkinson. Relat. Disord. 2015;21:1269–1272. doi: 10.1016/j.parkreldis.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Bansal K, et al. A pilot study to understand the relationship between cortical arousals and leg movements during sleep. Sci. Rep. 2022;12:12685. doi: 10.1038/s41598-022-16697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taebi A, Solar BE, Bomar AJ, Sandler RH, Mansy HA. Recent advances in seismocardiography. Vibration. 2019;2:64–86. doi: 10.3390/vibration2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ode KL, et al. A jerk-based algorithm ACCEL for the accurate classification of sleep-wake states from arm acceleration. iScience. 2022;25:103727. doi: 10.1016/j.isci.2021.103727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.