Abstract
We examined the potential for the development of fluoroquinolone resistance in Neisseria meningitidis by cultivating two clinical isolates of meningococci in the presence of concentrations of ciprofloxacin at and about the predetermined MIC. The quinolone resistance determining regions (QRDRs) of gyrA and parC of 50 stable quinolone-resistant mutants derived in vitro were sequenced and compared with QRDR alterations reported in clinical isolates of quinolone-resistant meningococci and gonococci. MICs to ciprofloxacin and trovafloxacin were determined and sequence changes were correlated with quinolone MICs. Ciprofloxacin and trovafloxacin MICs of the in vitro-derived quinolone-resistant mutants ranged up to 16 mg/liter. Single GyrA alterations were the first change detected and were accompanied by raised MICs, followed by double GyrA changes and still higher MICs. MICs increased further as single ParC substitutions appeared and these were always in the presence of a single or double GyrA change. GyrA changes occurred at positions 91 and 95 with substitutions of Asp-95→Asn and Thr-91→Ala and Ile. Changes in the parC QRDR occurred at positions 85, 86, and 91 with four substitutions, Gly-85→Asp, Asp-86→Asn, Glu-91→Gly, and Glu-91→Lys, detected. The nature of the individual QRDR substitution appeared to influence the level of quinolone resistance expressed, and this varied with the quinolone agent examined. Close similarities occurred between the sequence and nature of QRDR changes in clinical and in vitro-generated quinolone-resistant mutants and with those previously reported for clinical and in vitro-generated quinolone-resistant gonococci. This suggests that quinolone resistance in meningococci may arise in the same manner and reach similar levels in vivo to those seen in quinolone-resistant Neisseria gonorrhoeae.
Fluoroquinolone antibiotics are highly active against Neisseria meningitidis in vitro but are rarely used for the treatment of invasive meningococcal disease. Parenterally administered penicillins remain the recommended treatment in septicaemic invasive meningococcal disease, and the mainly oral route of quinolone administration and their restricted role in children have meant that their place in the treatment of bacterial meningitis has been limited (24). However, fluoroquinolones are recommended as chemoprophylactic agents for the prevention of invasive meningococcal disease (4), and high rates of clearance of Neisseria meningitidis from the oropharynx have been observed with single-dose ciprofloxacin (24).
Meninogococcal resistance to the fluoroquinolones has been rarely reported, however, and that only at a low level. A study in Greece revealed a single isolate from 472 strains from military recruits was resistant to ciprofloxacin at an MIC of ≥1 mg/liter (25). Clinical isolates of meningococci with raised MICs to ciprofloxacin were isolated in England and Wales after 1995, but their prevalence and MIC range were not reported (E. B. Kaczmarski, S. J. Gray, A. D. Carr and R. H. Mallard. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1466, 1999). The few reports available detail low-level resistance with ciprofloxacin MICs of 0.12 and 0.25 mg/liter. Examples are a serogroup B meningococcus isolated from a mucosal site of a client at a sexually transmitted diseases clinic in France (I. Casin, B. Gandry, F. Lassau, M. Janier, P. Lagrange, and E. Collatz, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2101, 1999), an invasive serogroup C meningococcus from Australia (T. R. Shultz, J. W. Tapsall, P. A. White, and P. J. Newton, letter, Antimicrob. Agents Chemother. 44:1116, 2000), and a serogroup B meningococcus isolated in Spain from CSF (B. Alcala, C. Saledo, L. de la Fuente, L. Arreaza, M. J. Uria, R. Abad, R. Enriquez, J. A. Vazquez, M. Motge, and J. de Batlle., correspondence, J. Antimicrob. Chemother. 53:409-410, 2004).
In contrast, there is now widespread resistance to the fluoroquinolones in the closely related Neisseria gonorrhoeae in many parts of the world, with ciprofloxacin MICs of 32 mg/liter and higher reported (10, 19, 23). The mechanisms of fluoroquinolone resistance in N. gonorrhoeae have been investigated for some time. Early studies by Belland et al., using fluoroquinolone-resistant gonococci derived in vitro, described point mutations in the quinolone-resistance determining regions (QRDRs) of the target sites for the fluoroquinolones, namely, the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV (2). They established that GyrA was the primary site of action for fluoroquinolones on gonococci and ParC was a secondary target and that multiple QRDR changes were accompanied by increases in resistance as measured by MIC determinations (6). The in vitro model proposed by Belland et al. for the development of fluoroquinolone resistance in gonococci was subsequently shown to be indicative of the QRDR changes that developed in clinical isolates of fluoroquinolone-resistant gonococci (5, 16). More recently, the contribution of efflux mechanisms to fluoroquinolone resistance in gonococci has been further elucidated (7).
The QRDR changes underlying fluoroquinolone resistance in N. meningitidis have been little studied, however. The mucosal isolate from France had an Asp-95→Gly GyrA change, the invasive meningococcus from Australia an Asp-95→Asn GyrA change, and the Spanish strain had a Thr-91→Ile GyrA alteration. Threonine at position 91 in meningococci was equivalent to the amino acid serine found in gonococci when a comparison of the nucleotide sequences was undertaken (GenBank/EMBL database search). The earlier reports from France and Australia noted that the GyrA alterations observed in the fluoroquinolone-resistant meningococci were also present in fluoroquinolone-resistant gonococci. In addition, the GyrA change (Thr91→Ile) in the Spanish isolate has an equivalent substitution described in resistant N. gonorrhoeae (Ser91→Ile) (15). There are thus parallels in the development of fluoroquinolone resistance mediated by QRDR changes in the pathogenic neisseriae, although to date, no ParC changes have been observed in meningococci.
The emergence of fluoroquinolone-resistant pneumococci and oral streptococci, mediated in part at least by QRDR changes, has been linked to exposure to fluoroquinolones as the use of these antibiotics for the treatment of respiratory disease increases (8, 9). It is reasonable to assume that oropharyngeal meningococci may be similarly exposed to fluoroquinolones and also undergo QRDR alterations over time. In this study, the potential for development of fluoroquinolone resistance mediated by GyrA and ParC sequence alteration in Neisseria meningitidis was explored using an in vitro approach, similar to that used by Belland et al. (2) for predicting QRDR changes in gonococci. The sequence changes occurring in the QRDR of in vitro-derived fluoroquinolone-resistant meningococci were determined, and the effects of these alterations were correlated to resistance to ciprofloxacin and trovafloxacin by MIC determinations. These GyrA and ParC alterations were also compared with events known to occur in equivalent positions of the QRDR of clinical isolates of fluoroquinolone-resistant gonococci.
(This material was presented in part at the Twelfth International Pathogenic Neisseria Conference, Galveston, TX, 12 to 17 November 2000 [T.R. Shultz and J. Tapsall, abstract 217].)
MATERIALS AND METHODS
Meningococcal isolates used for in vitro generation of resistance.
Two meningococci were studied. A ciprofloxacin-sensitive N. meningitidis, AN-JA was grown from the urethral discharge of a male patient attending a sexually transmitted diseases clinic in Sydney, Australia. This isolate was chosen for study because it exhibited an ability to consistently produce colonies within the zone of inhibition around a 1 μg disk of ciprofloxacin. The isolate, characterized by standard procedures including carbohydrate utilization, serogroup, serotype, and serosubtype determinations, was of the phenotype C:2a:P1.5. A second isolate, TH-AN, from a case of invasive meningococcal disease and showing decreased quinolone susceptibility, has been previously described (T. R. Shultz, J. W. Tapsall, P. A. White, and P. J. Newton, Letter, Antimicrob. Agents Chemother. 44:1116, 2000).
Antibiotic susceptibility testing.
Antibiotic susceptibility testing for ciprofloxacin and trovafloxacin were performed as described earlier (16). Briefly, an inoculum of 104 CFU per spot was applied to Isosensitest agar (Oxoid, Basingstoke, United Kingdom) containing 8% saponin-lysed horse blood and doubling dilutions of antibiotic and incubated in an atmosphere of 5% CO2 in air for 24 h. As an adjunct to this conventional doubling dilution series, more precise MICs were obtained for ciprofloxacin only by using additional intermediate concentrations of antibiotic (intermediate dilution series [IDS]). For the purposes of this study, the following interpretation of MICs, derived from the National Neisseria Network of Australia (19), was applied: ciprofloxacin sensitive strains were those with MICs less than or equal to 0.03 mg/liter, ciprofloxacin less sensitive strains had MICs in the range 0.045 to 0.75 mg/liter, ciprofloxacin resistant strains had MICs between 1 and 2 mg/liter, and high-level ciprofloxacin resistance referred to MICs greater than 2 mg/liter. The antibiotic powders were of defined potency and gifts from Bayer (ciprofloxacin) and Pfizer (trovafloxacin) Pharmaceuticals. Gonococcal isolates used in the World Health Organization GASP quality assurance surveys (21) were included in the testing as controls. World Health Organization C had a ciprofloxacin MIC of 0.016 mg/liter, World Health Organization H had a MIC of 2 mg/liter and World Health Organization I had a MIC of 0.5 mg/liter by the test method used.
In vitro generation of quinolone resistance.
In vitro generation of quinolone resistance was performed by use of GC agar plates (Acumedia, Baltimore, Maryland), supplemented with 8% saponin-lysed horse blood and containing ciprofloxacin concentrations one and two doubling dilutions above and below the previously determined MIC. Each plate received 32 inocula of each organism at a concentration of 106 CFU/spot. Inoculated plates were incubated in 5% CO2 in air at 37°C and examined for growth at 24, 48 and 72 h. Any colonies that grew on the plates containing ciprofloxacin at or above the MIC were enumerated. The stability of these variant colonies was assessed by serial subculture onto GC agar with and without ciprofloxacin for 8 days and the stable variants were stored at −70°C. The frequency of mutation rate was calculated by averaging the total number of mutant colonies present, multiplied by the dilution factor. These cycles were repeated with in vitro derived mutants exhibiting progressively higher MICs to obtain up to four successive generations of such mutants. The MICs of each stable variant (see Tables 3 and 4) were determined prior to the attempt to generate further mutants with still higher MICs, again by inoculation onto plates containing antibiotic concentrations, as described above.
TABLE 3.
Correlation of QRDR changes with ciprofloxacin and trovafloxacin activity on the quinolone-sensitive Neisseria meningitidis AN-JA and 44 in vitro-generated mutants with decreased quinolone sensitivity
| Strain code | Lineage | MIC (mg/liter)a
|
Differenceb | QRDR alteration(s)
|
||
|---|---|---|---|---|---|---|
| int cip | tva | GyrA | ParC | |||
| AN-JA | Parent | 0.016 | 0.03 | +1 | nil | Nil |
| V2 | F1 | 0.125 | 0.06 | −1 | Asp95Asn | Not tested |
| V1 | F1 | 0.38 | 0.25 | −0.5 | Thr91Ile | Nil |
| V6, V10, V12 | 0.38 | 0.25 | −0.5 | Thr91Ile | Nil | |
| V7 | V1, F2 | 0.38 | 0.125 | −1.5 | Thr91Ile | Nil |
| V20 | 0.38 | 0.5 | +0.5 | Thr91Ile | Nil | |
| V14 | V2, F2 | 0.38 | 0.25 | −0.5 | Thr91Ala, Asp95Asn | Nil |
| V22 | 0.38 | 0.25 | −0.5 | Thr91Ile, Asp95Asn | Nil | |
| V5 and V15 | V1, F2 | 0.5 | 0.25 | −1 | Thr91Ile | Nil |
| V17 | 0.5 | 1 | +1 | Thr91Ile | Nil | |
| V16 and V21 | 0.75 | 0.5 | −0.5 | Thr91Ile | Nil | |
| V18 | V1, F2 | 0.75 | 0.25 | −1.5 | Thr91Ile | Nil |
| V4, V8, V9, V13 | 0.75 | 0.5 | −0.5 | Thr91Ile | Glu91Gly | |
| V11 and V19 | 0.75 | 1 | +0.5 | Thr91Ile | Asp86Asn | |
| V23 | V2, F2 | 0.75 | 0.5 | −0.5 | Thr91Ile, Asp95Asn | Nil |
| V25, V26, V30 | V1, F3 | 3 | 2 | −0.5 | Thr91Ile, Asp95Asn | Glu91Gly |
| V28 and V33 | 3 | 4 | +0.5 | Thr91Ile, Asp95Asn | Asp86Asn | |
| V39 | V2, F3 | 4 | 2 | −1 | Thr91Ile, Asp95Asn | Glu91Gly |
| V37 | V2, F3 | 6 | 16 | +1.5 | Thr91Ile, Asp95Asn | Gly85Asp |
| V38 | 6 | 4 | −0.5 | Thr91Ile, Asp95Asn | Glu91Lys | |
| V41 | V1, F4 | 6 | 2 | −1.5 | Thr91Ile, Asp95Asn | Glu91Gly |
| V54 | V2, F4 | 6 | 4 | −0.5 | Thr91Ile, Asp95Asn | Glu91Gly |
| V42 and V43 | V1, F4 | 8 | 2 | −2 | Thr91Ile, Asp95Asn | Glu91Gly |
| V48 | 8 | 4 | −1 | Thr91Ile, Asp95Asn | Glu91Gly | |
| V47 | 8 | 8 | 0 | Thr91Ile, Asp95Asn | Asp86Asn | |
| V44 and V45 | V1, F4 | 12 | 4 | −1.5 | Thr91Ile, Asp95Asn | Glu91Gly |
| V46 | 12 | 4 | −1.5 | Thr91Ile, Asp95Asn | Asp86Asn | |
| V53 | V2, F4 | 12 | 8 | −0.5 | Thr91Ile, Asp95Asn | Glu91Lys |
| V49 | V1, F4 | 16 | 4 | −2 | Thr91Ile, Asp95Asn | Glu91Gly |
| V50 and V51 | V2, F4 | 16 | 16 | 0 | Thr91Ile, Asp95Asn | Gly85Asp |
| V52 | 16 | 8 | −1 | Thr91Ile, Asp95Asn | Glu91Lys | |
int cip, intermediate ciprofloxacin MICs, IDS; tva, trovafloxacin MICs, doubling dilution series.
Fold difference in activity between cip and tva.
TABLE 4.
Correlation of QRDR changes with ciprofloxacin and trovafloxacin activity on the less quinolone sensitive Neisseria meningitidis TH-AN and six in vitro-derived mutants with decreaseda quinolone sensitivity
| Strain code | Lineage | MIC (mg/liter)
|
Difference | QRDR alterations
|
Reference | ||
|---|---|---|---|---|---|---|---|
| int cip | tva | GyrA | ParC | ||||
| TH-AN | parent | 0.09 | 0.06 | −0.5 | Asp95Asn | Nil | 11 |
| T1 | F1 | 0.38 | 0.125 | −1.5 | Asp95Asn | Nil | |
| T2 | 0.5 | 0.125 | −2 | Asp95Asn | Nil | ||
| T3, T4, T6 | F2 | 0.75 | 0.125 | −2.5 | Asp95Asn | Nil | |
| T5 | 1 | 0.125 | −3 | Asp95Asn | Nil | ||
See Table 3, footnotes a and b.
Molecular sequencing of the gyrA and parC QRDRs.
QRDRs from three ciprofloxacin-sensitive meningococci were also amplified and sequenced, AN-JA (sensitive parent strain) and two control strains, L1810 (C:2a:P1.2) and L2057 (B:4:P1.5). Sequencing the QRDRs of gyrA and parC followed closely the methods previously described and used for QRNG and for quinolone less sensitive meningococci (16, 18). DNA extraction was performed by boiling about a pin-head amount of bacteria suspended in 30 μl of distilled water for five minutes followed by storage at 4°C. Primers for gyrA QRDR were those designed by Deguchi et al. for gonococci and yielded a 225-bp fragment (5). The 225-bp parC QRDR was amplified using a forward primer as described previously by Belland et al. for gonococci and a reverse primer based on the work of Trees et al. (2, 22). PCR amplification consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s.
DNA sequencing and computer analysis.
PCR products were purified by a polyethylene glycol 6000 (BDH, Poole, England) precipitation and washed with 70% ethanol. Products were sequenced directly using 4 μl of template (≈100 ng), 1 μl (10 pmol) of primer, 10 μl of water, 2.5 μl of CSA buffer (Tris 0.05 M, pH 9.0, 1 M MgCl2) and 2.5 μl of dye terminator premix (Perkin-Elmer). Following thermal cycling, reactions were precipitated and analyzed using an ABI 377 DNA sequencer (Perkin-Elmer Applied Biosystems). GenBank and EMBL database searches from the Australian National Genomic Information Service (http://www.angis.org.au) were conducted using BLAST and pair-wise alignments of DNA sequences were carried out using the GCG program GAP.
RESULTS
A total of 50 stable mutants were derived in vitro after exposure to ciprofloxacin. Forty-four resistant mutants were generated from the parent strain, AN-JA, and six mutants from TH-AN. These mutants exhibited different fluoroquinolone MICs and/or different QRDR changes (Tables 1 to 234).
TABLE 1.
Lineage and mutant frequency of the quinolone-resistant variant strains generated in vitro from the parent strain AN-JA
| Strain code | Lineage | Frequency of mutations (CFU/spot) |
|---|---|---|
| AN-JA | Parent | |
| V1 | F1 | 1 × 10−6 |
| V4 | F2 | 2 × 10−6 |
| V25 | F3 | 2 × 10−5 |
| V41, V42, V43 | F4 | |
| V5 | F2 | 1 × 10−6 |
| V6 | F2 | 1 × 10−6 |
| V7 | F2 | 1 × 10−6 |
| V8 | F2 | 1 × 10−6 |
| V26 | F3 | 1 × 10−6 |
| V44 and V45 | F4 | |
| V9 | F2 | 1 × 10−6 |
| V10 | F2 | 1 × 10−6 |
| V11 | F2 | 1 × 10−6 |
| V28 | F3 | 2 × 10−7 |
| V46 and V47 | F4 | |
| V12 | F2 | 1 × 10−6 |
| V13 | F2 | 1 × 10−6 |
| V30 | F3 | 1 × 10−6 |
| V48 and V49 | F4 | |
| V15 | F2 | 1 × 10−6 |
| V16 | F2 | 1 × 10−6 |
| V17 | F2 | 1 × 10−6 |
| V18 | F2 | 1 × 10−6 |
| V19 | F2 | 1 × 10−6 |
| V33 | F3 | |
| V20 | F2 | 1 × 10−6 |
| V21 | F2 | 1 × 10−6 |
| V2 | F1 | 2 × 10−6 |
| V14 | F2 | |
| V22 | F2 | 6 × 10−8 |
| V37 | F3 | 8 × 10−8 |
| V50 and V51 | F4 | |
| V38 | F3 | 8 × 10−8 |
| V52 and V53 | F4 | |
| V23 | F2 | 6 × 10−8 |
| V39 | F3 | 9 × 10−8 |
| V54 | F4 |
TABLE 2.
Lineage and mutant frequency of the quinolone-resistant variant strains generated in vitro from the parent strain TH-AN
| Strain code | Lineage | Frequency |
|---|---|---|
| TH-AN | Parent | 1 × 10−6 |
| T1 | F1 | 1 × 10−6 |
| T3 and T4 | F2 | |
| T2 | F1 | 1 × 10−6 |
| T5 and T6 | F2 |
PCR amplification and sequencing of gyrA and parC of the ciprofloxacin-susceptible parent strain AN-JA and the two control strains, L1810 and L2057 (ciprofloxacin MIC-double dilution series, <0.03 mg/liter) yielded a PCR product of the expected size. The resultant sequences were compared to database information on the following meningococci, NMN 3 and Z2491 (serogroup A), GNMCZ78F (serogroup C), MC58 (serogroup B), and NMN1 to establish a baseline or unchanged sequence for the QRDR. Some limited variation occurs in gyrA and parC between meningococci from different serogroups, but these changes tended to be outside of the QRDR and none have been associated with resistance. None of the changes associated with resistance observed in this study were seen in any of the control sequences analyzed.
Table 3 shows, in ascending order of ciprofloxacin MICs, details of QRDR alterations in the 44 in vitro-generated mutants from the quinolone-susceptible parent strain AN-JA. Ciprofloxacin MICs increased from 0.016 mg/liter to 16 mg/liter (IDS) (strains V49 to V52) over four cycles with a mutation frequency that ranged between 2 × 10−5 and 9 × 10−8 (Tables 1 and 3). Increases in trovafloxacin MICs were from 0.03 to 16 mg/liter (double dilution series) (strains V37, V50, and V51) over the same four cycles (Table 3). Over two induction cycles (Table 4) with the quinolone-resistant parent strain TH-AN containing the single GyrA alteration, Asp-95→Asn, ciprofloxacin MICs rose from 0.09 mg/liter (IDS) to 1 mg/liter (strain T5) and trovafloxacin MICs doubled from 0.06 to 0.125 mg/liter over the same two cycles. No additional QRDR changes were accompanied by these MIC increases.
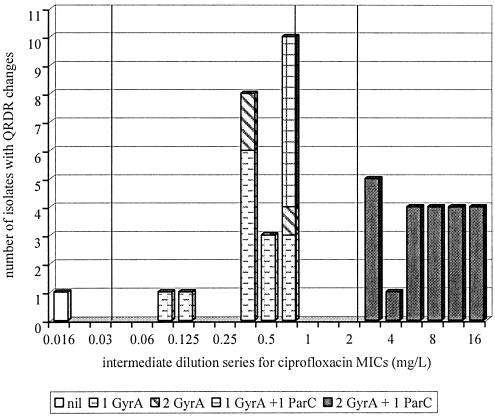
In the resistant variant meningococci (ciprofloxacin MIC-double dilution series, >0.03 mg/liter) generated from AN-JA, a sequence of QRDR changes was observed (16). Single point mutations in gyrA was followed by double GyrA or single GyrA changes in conjunction with a single ParC change (Fig. 1). The accompanying ciprofloxacin MICs (IDS) associated with these changes were in the range 0.125 (strain V2) to 0.75 mg/liter (strains V4 to V23). The final change observed was the presence of two GyrA and single ParC substitutions, which saw ciprofloxacin MICs increase substantially to between 3 and 16 mg/liter (IDS) (Table 3).
FIG. 1.
Quinolone-resistant meningococci shown by QRDR change and ciprofloxacin MIC for strain AN-JA and its four generations of resistant variants and the parent strain TH-AN. The vertical lines on the graph represent the range of MICs for each category of fluoroquinolone resistance according to the criteria of the National Neisseria Network, Australia. These MICs have been adjusted to suit the single MIC assays performed (IDS) in this study so that the following applies: ciprofloxacin-sensitive strains have MICs less than or equal to 0.03 mg/liter; less sensitive strains have MICs ranging from 0.045 to 0.75 mg/liter; resistant strains have MICs between 1 and 2 mg/liter; and strains with high-level resistance have MICs greater than or equal to 3 mg/liter.
Point mutations in the gyrA QRDR were found at positions 91 and 95 and in parC at positions 85, 86, and 91. The in vitro generation of resistant variants with QRDR changes were only obtained from one parent strain, AN-JA. Strains V1 and V2 were the two first generation mutants derived from this strain and had ciprofloxacin MICs of 0.38 and 0.125 mg/liter (IDS). These had single gyrA mutations of Thr-91→Ile and Asp-95→Asn, respectively (Table 3). The second cycle yielded a total of 20 mutants from these two strains, of which 17 were from strain V1 and had MICs between 0.38 and 0.75 mg/liter (IDS), and three strains from V2 that had MICs of 0.38 or 0.75 mg/liter (IDS). Six of the V1 lineage mutants had ParC changes in addition to the previous GyrA changes. Two of these six strains exhibited the ParC substitution Asp-86→Asn and four strains had the substitution Glu-91→Gly (Table 3). In contrast, those of the V2 lineage had a second GyrA change of Thr-91→Ile (V22) or Thr-91→Ala (V14) to accompany the previous GyrA change of Asp-95→Asn (Table 3).
The third-generation mutants were eight in number with ciprofloxacin MICs between 3 and 6 mg/liter (IDS). Five were of the V1 lineage and three originated from V2. All eight now contained the same double GyrA change (Thr-91→Ile and Asp-95→Asn) and one of four different ParC changes (Gly-85→Asp, Asp-86→Asn, Glu-91→Gly or Glu-91→Lys). The fourth-generation changes in fourteen strains saw MICs increased from 6 to 16 mg/liter (IDS) for ciprofloxacin, but there were no further QRDR alterations in either GyrA or ParC. It is likely that separate, but undetermined, mechanisms were responsible for this increased resistance and these fourth generation variants are not further considered in the following analysis.
The nature of the substitutions in GyrA and ParC appeared to influence the ciprofloxacin MICs observed (Table 3, Fig. 1). When it occurred as a single change, the substitution of aspartic acid to asparagine at position 95 in GyrA conferred only lower levels of ciprofloxacin resistance (ciprofloxacin MIC-IDS, 0.125 mg/liter) (strain V2). In contrast, GyrA changes in position 91 of strain V1 resulted in MICs around one and a half fold higher than Asp95 changes (ciprofloxacin MIC-IDS, 0.38 mg/liter). ParC changes appeared to influence ciprofloxacin MICs to a greater extent when coupled with a dual GyrA change, as shown by comparisons of precise MICs for mutants belonging to the same lineage, V22 and V37 (MIC-IDS, 0.38 and 6 mg/liter) and V23 and V39 (MIC-IDS, 0.75 and 4 mg/liter), for instance (Table 3).
The nature of the QRDR changes affected the relative activity of ciprofloxacin and trovafloxacin when results from the doubling dilution series (DDS) were compared (Table 3, Fig. 2). In the absence of QRDR changes in the AN-JA parent, trovafloxacin was less active weight for weight than ciprofloxacin. However, when QRDR alterations were present, the relative activity of trovafloxacin altered. Those variants containing single GyrA changes gave a range of trovafloxacin MICs between one and a half fold less and up to one-fold greater than those for ciprofloxacin (Table 3, Fig. 2). Variant strains with combined single GyrA and ParC changes (strains V4, V8, V9, V11, V13, and V19) or double GyrA changes (strains V14, V22, and V23) had ciprofloxacin and trovafloxacin MICs at similar levels. The greatest relative increase in trovafloxacin activity was evident among those variant strains containing double GyrA changes coupled with single ParC changes. The relative activities varied however, and appeared to be dependent on the individual amino acid substitutions present. For example, strains containing the ParC substitution Glu-91→Gly had trovafloxacin MICs up to onefold less than those for ciprofloxacin (strains V25, V26, V30, and V39). Alternatively, strains containing a different ParC substitution, such as Gly-85→Asp, had trovafloxacin MICs 1.5-fold higher than those for ciprofloxacin (strain V37) (Table 3, Fig. 2).
FIG. 2.
Activity of trovafloxacin relative to ciprofloxacin (measured as the difference in MICs using the doubling dilution series) in the three generation of resistant variants derived from the parent strain AN-JA and containing a variety of QRDR alterations.
DISCUSSION
The in vitro generation of quinolone-resistant mutants of N. meningitidis demonstrated the potential for the further development of fluoroquinolone resistance in meningococci when variants with ciprofloxacin MICs up to 16 mg/liter were obtained following serial passage on ciprofloxacin-containing medium. The importance of QRDR changes and in particular those arising from gyrA substitutions was also demonstrated.
To date, only GyrA changes have been reported in clinical isolates of resistant meningococci and these in positions 91 (Thr-91→Ile) and 95 (Asp-95→Asn and Asp-95→Gly). Two of these three amino acid substitutions (Thr-91→Ile and Asp-95→Asn) found in clinical strains were also present in in vitro-derived mutants, but the other change, Asp-95→Gly, was not. An additional in vitro-derived substitution, Thr-91→Ala, has not as yet been reported in clinical isolates. ParC QRDR alterations were found in meningococci for the first time, demonstrating the potential for this change to occur in vivo and thus for the development of higher levels of fluoroquinolone resistance. ParC alterations occurred subsequent to alterations in GyrA. Four substitutions, Gly-85→Asp, Asp-86→Asn, Glu-91→Gly, and Glu-91→Lys, were generated at three positions in the QRDR.
Of relevance are the close similarities between the QRDR changes observed in meningococci and gonococci. All of the QRDR amino acid substitutions found in clinical isolates and the in vitro-generated fluoroquinolone-resistant meningococci have been reported in gonococci. Of the GyrA changes detected thus far in clinical isolates of meningococci, Asp-95→Asn and Asp-95→Gly were common findings in clinical isolates of fluoroquinolone-resistant gonococci (16, 23), with the latter change often associated with multiple QRDR alterations. The GyrA substitution in meningococci at position 91 (Thr-91→Ile) was equivalent to the substitution Ser91→Ile recently reported in fluoroquinolone-resistant gonococci from Japan (15). The site of the parC alterations and the substitutions generated in vitro in the fluoroquinolone-resistant meningococci were the same as those documented in clinical isolates of fluoroquinolone-resistant gonococci (23). However, ParC changes were not found at positions 87 and 88 in the QRDR of meningococci. ParC changes at these positions were commonly reported in fluoroquinolone-resistant gonococci and were associated with high-level ciprofloxacin resistance (18).
A further parallel noted between gonococci and meningococci was the effect of both the sequence and nature of the GyrA and ParC substitutions on ciprofloxacin MICs. Single nucleotide mutations were acquired sequentially in the two target site enzymes, GyrA and ParC. A change in gyrA initiated phenotypic fluoroquinolone resistance. ParC changes always appeared after GyrA changes, and the successive changes in the QRDRs resulted in progressive increases in MICs. The highest MICs in in vitro-derived meningococci occurred in the presence of double GyrA changes accompanied by a single ParC change. This was the maximum number of changes generated in this study in meningococci, whereas double changes in both GyrA and ParC were reported in clinical isolates of fluoroquinolone-resistant gonococci, and this reflects the fuller extent of their development of resistance by this mechanism.
The nature of the amino acid changes also influenced the levels and the development of fluoroquinolone resistance. Some examples include the amino acid change in GyrA of variant strain V1 at position 91 (Thr91), which resulted in a higher MIC than the change in V2 at position 95 (Asp95). Further, the change Thr91 was required before an alteration in parC could occur. ParC changes had a greater effect on MIC levels in the presence of a double GyrA change. The nature and sequence of GyrA and ParC changes also affected the relative activities of ciprofloxacin and trovafloxacin, a phenomenon observed also in gonococci and other genera (16, 26).
Despite the shared evolutionary path in the two pathogenic neisseriae, the meningococcus is more often susceptible to antibiotics. The gonococcus has now developed in vivo the high-level fluoroquinolone resistance revealed by earlier in vitro studies (2, 6, 18, 23). With the penicillins, beta-lactamase-producing meningococci are a rare event and chromosomally mediated resistance occurs less frequently in meningococci and the MICs observed are generally lower than in gonococci (1). Antignac et al. have suggested that differences in gonococci and meningococci arose in the gene encoding penicillin binding protein 2 (PBP-2) after a separation event of the two species and that this helps to account for the different levels of penicillin resistance observed (1). Further, the full development of chromosomally mediated resistance to penicillin in gonococci requires the aggregation of a number of changes at various loci that have not been observed in meningococci (13). It is thus possible that some further genomic differentiation may be found, either in the QRDR of gonococci and meningococci or elsewhere, which will account for differences in the development of fluoroquinolone resistance in the two species.
This study has a number of limitations. It concentrated on the examination of target site changes occurring in the gyrA and parC QRDRs, but increments in MICs to ciprofloxacin were observed that were unaccompanied by alterations in these genes. This suggests either that the QRDR extends beyond the areas currently defined, that additional mechanisms of resistance may exist, or that changes in the other topoisomerase subunits, gyrB and parE, may be involved. However, changes in gyrB and parE are rare (12). In studies where changes in gyrB and parE were sought in gonococci, gyrB changes were absent (10) or accompanied by low-level resistance only (17), and parE changes, found in a single study only, did not alter ciprofloxacin MICs (10).
Altered outer membrane permeability and antibiotic efflux are also recognized as mechanisms contributing to quinolone resistance in the neisseriae. Multiple efflux pumps are described in gonococci, and a NorM efflux pump was described in both meningococci and gonococci (14). However, further clarification of the role of these mechanisms is required. Some are of the opinion that efflux mechanisms play a lesser role in meningococci than in gonococci (11), and the NorM pump had minimal effect on ciprofloxacin MICs (14). Also in this context, the change Gly85→Asp was of additional interest because it was positioned outside the region commonly accepted as the QRDR. In this instance, the ParC change was accompanied by two GyrA changes, and together these conferred relatively high-level ciprofloxacin resistance (MICs, 6 mg/liter). The clarification of the molecular basis of these mechanisms in these and other studies may assist the development of molecular techniques for non-culture-based detection of fluoroquinolone resistance. Non-culture-based diagnosis for invasive meningococcal disease is increasingly important, but nucleic acid amplification assay technology does not currently permit a full and proper assessment of antimicrobial resistance in neisseriae.
The hypermutable strain used here may not be generally representative of meningococci. However, the value of hypermutable strains to assess antimicrobial resistance to novel drug candidates or to induce rare mutations has been shown (A. J. O'Neill and I. Chopra, letter, Antimicrob. Agents Chemother. 45:1599-1600, 2001). Although QRDR mutations were relatively easy to induce and were stable after many generations, they may also be deficient in secondary fitness compensatory mutations required for successful existence in vivo (3). Further, additional QRDR changes were not found in strain TH-AN despite the presence of an existing GyrA change, suggesting the possibility of strain-to-strain variation in the potential for resistance development. Similar findings have been reported in vitro studies of generation of fluoroquinolone resistance in gonococci (2, 20).
The presence of identical GyrA QRDR changes in both clinically and in vitro derived fluoroquinolone-resistant meningococci, however, provides some reassurance on the relevance of the in vitro observations reported here, as do the similarities noted between the sequence and nature of gyrA and parC mutations in meningococci and Neisseria gonorrhoeae. This study suggests that quinolone resistance conferred through gyrA and parC mutations is likely to continue to emerge in N. meningitidis as it has in N. gonorrhoeae. In particular, the generation of alterations in ParC of meningococci, not previously reported, has implications for the development of resistance in vivo. Any potential for increasing fluoroquinolone resistance in meningococci may be realized sooner by the exposure of meningococci to fluoroquinolones in vivo, and parallel the emergence of fluoroquinolone-resistant pneumococci and oral streptococci consequent upon increasing use of these agents in respiratory disease.
REFERENCES
- 1.Antignac, A., P. Kriz, G. Tzanakaki, J-M Alonso, and M-K Taha. 2001. Polymorphisms of Neisseria meningitidis penA gene associated with reduced susceptibility to penicillin. J. Antimicrob. Chemother. 47:285-296. [DOI] [PubMed] [Google Scholar]
- 2.Belland, R. J., S. G. Morrison, C. Ison, and W. M. Huang. 1994. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol. Microbiol. 14:371-380. [DOI] [PubMed] [Google Scholar]
- 3.Björkman, J., I. Nagaev. O. G. Berg, D. Hughes, and D. I. Anderson. 2000. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science. 287:1479-1482. [DOI] [PubMed] [Google Scholar]
- 4.Communicable Disease Network of Australia. 2001. Guidelines for the early clinical and public health management of meningococcal disease in Australia. Commonwealth Department of Health and Aged Care, Canberra, Australia.
- 5.Deguchi, T., M. Yasuda, M. Asano, K. Tada, H. Iwata, H. Komeda, T. Ezaki, I. Saito, and Y. Kawada. 1995. DNA gyrase mutations in quinolone-resistant clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 39:561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deguchi, T., M. Yasuda, M. Nakano, S. Ozeki, T. Ezaki, I. Saito, and Y. Kawada. 1996. Quinolone-resistant Neisseria gonorrhoeae: Correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob. Agents Chemother. 40:1020-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewi, B. E., S. Akira, H. Hayashi, and W. Ba-Thein. 2004. High occurrence of simultaneous mutations in target enzyme and MtrRCDE efflux system in quinolone-resistant Neisseria gonorrhoeae. Sex. Transm. Dis. 31:353-359. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos, G. M. 2004. Quinolone resistance mechanisms in pneumococci. Clin. Infect. Dis. 38(Suppl. 4):S350-356. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko, A., J. Sasaki, M. Shimadzu, A. Kanayama, T. Saika, and I. Kobayashi. 2000. Comparison of gyrA and parC mutations and resistance levels among fluoroquinolone-resistant isolates and laboratory-derived mutants of oral streptococci. J. Antimicrob. Chemother. 45:771-775. [DOI] [PubMed] [Google Scholar]
- 10.Lindback, E., M. Rahman, S. Jalal, and B. Wretlind. 2002. Mutations in gyrA, gyrB, parC and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS 110:651-657. [DOI] [PubMed] [Google Scholar]
- 11.Orus, P., and M. Vinas. 2001. Mechanisms other than penicillin-binding protein-2 alterations may contribute to moderate penicillin resistance in Neisseria meningitidis. Inter. J. Antimicrob. Agents 18:113-119. [DOI] [PubMed] [Google Scholar]
- 12.Pidcock, L. J. V. 1998. Fluoroquinolone resistance. Br. Med. J. 317:1029-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ropp, P. A., M. Hei, M. Olesky, and R. A. Nicholas. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roquette-Loughlin, C., S. A. Dunham, M. Kuhn, J. T. Balthazar, and W. W. Shafer. 2003. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J. Bacteriol. 185:1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shigemura, K., T. Shirakawa, H. Okada, N. Hinata, B. Acharya, S. Kinoshita, T. Kofuku, M. Kawabata, S. Kamidono, S. Arakawa, and A. Gotoh. 2004. Mutations in the gyrA and parC genes and in vitro activities of fluoroquinolones in 91 clinical isolates of Neisseria gonorrhoeae in Japan. Sex. Transm. Dis. 31:180-184. [DOI] [PubMed] [Google Scholar]
- 16.Shultz, T. R., J. W. Tapsall, and P. A. White. 2001. Correlation of in vitro susceptibilities to newer quinolones of naturally occurring quinolone-resistant Neisseria gonorrhoeae strains with changes in GyrA and ParC. Antimicrob. Agents Chemother. 45:734-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein, D. C., R. J. Danaher, and T. M. Cook. 1991. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 35:622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka, M., H. Nakayama, M. Haraoka, T. Saika, I. Kobayashi, and S. Naito. 2000. Susceptibilities of Neisseria gonorrhoeae isolates containing amino acid substitutions in GyrA, with or without substitutions in ParC, to newer fluoroquinolones and other antibiotics. Antimicrob. Agents Chemother. 44:192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tapsall, J. W., E. A. Phillips, and Y. M. Cossins. 1984. Penicillin sensitivity of gonococci in Australia: the development of an Australian gonococcal surveillance programme. Br. J. Vener. Dis. 60:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapsall, J. W., T. R. Shultz, and E. A. Phillips. 1992. Characteristics of Neisseria gonorrhoeae isolated in Australia showing decreased sensitivity to Quinolone antibiotics. Pathology. 24:27-31. [DOI] [PubMed] [Google Scholar]
- 21.The W.H.O. Western Pacific Gonococcal Antimicrobial Surveillance Programme. 2002. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the W.H.O. Western Pacific Region, 2001. Commun. Dis. Intelligence. 26:541-545. [PubMed] [Google Scholar]
- 22.Trees, D. L., A. L. Sandul, W. L. Whittington, and J. S. Knapp. 1998. Identification of novel mutation patterns in the parC gene of ciprofloxacin-resistant isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 42:2103-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trees, D. L., A. L. Sandul, V. Peto-Mesola, M. R. Aplasca, H. B. Leng, W. L. Whittington, and J. S. Knapp. 1999. Alterations within the quinolone resistance-determining regions of GyrA and ParC of Neisseria gonorrhoeae isolated in the Far East and the United States. Inter. J. Antimicrob. Agents. 12:325-332. [DOI] [PubMed] [Google Scholar]
- 24.Tunkel, A. R., and W. M. Scheld. 1993. Treatment of bacterial meningitis, p. 381-393. In: Hooper, D. S., and J. S. Wolfson (ed.), Quinolone antimicrobial agents, 2nd ed.. American Society for Microbiology, Washington, D.C.
- 25.Tzanakaki, G., C. C. Blackwell, J. Ktremastinou, C. Kallergi, G. Kouppari, and D. M. Weir. 1992. Antibiotic sensitivities of Neisseria meningitidis isolates from patients and carriers in Greece. Epidemiol. Infect. 108:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigel, L. M., G. J. Anderson, R. R. Facklam, and F. C. Tenover. 2001. Genetic analyses of mutations contributing to fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:3517-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]