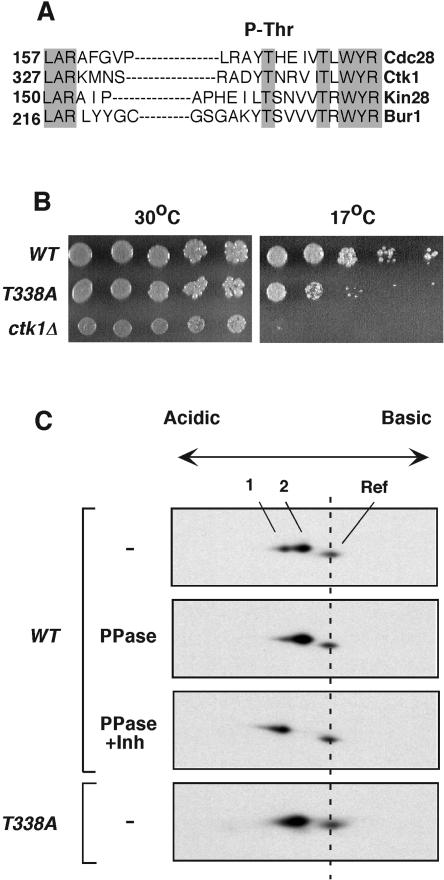
FIG. 1.
Ctk1 is phosphorylated on Thr-338. (A) Protein sequence alignment of T-loop regions in four S. cerevisiae CDKs. Grey shading denotes conserved residues. Numbers indicate positions of the T loops within the individual CDKs. The alignment was done using the Clustal algorithm. The site of activating phosphorylation is indicated at the top. (B) Serial dilutions of wild-type (WT) and ctk1T338A and ctk1Δ mutant cultures were spotted onto YPD plates. The plates were incubated at 30°C and at 17°C and photographed after 4 days. (C) Two-dimensional gel analysis of Ctk1. Yeast proteins were separated by nonequilibrium pH gel electrophoresis according to their charges and molecular weights. Ctk1-8DE-HA contained eight negatively charged amino acids to compensate for the large positive charge of the protein. Ctk1-8DE-HA was detected by immunoblotting with rabbit anti-HA antibodies. In wild-type yeast extracts, Ctk1-8DE-HA migrated as a doublet (spots 1 and 2, top). Yeast extracts were treated with λ phosphatase (PPase) in the absence (second panel) or presence (third panel) of phosphatase inhibitors (Inh). Ctk1T338A-8DE-HA is shown in the lower panel. A yeast extract containing Ctk1-HA with no added acidic residues was included in the loading mixtures to provide a reference (Ref) mark to monitor the positions of the Ctk1-8DE-HA spots. Ctk1-HA is more positively charged and smaller than Ctk1-8DE-HA. Ctk1-HA was not treated with phosphatase, and its position remained constant in the various experiments.