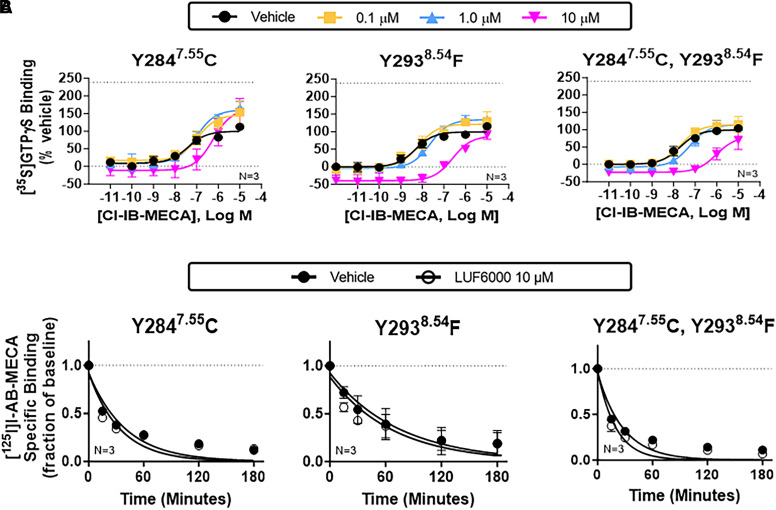
Fig. 6.
Characterization of Y2847.55C and Y2938.54F mutant receptors. [35S]GTPγS (A) and [125I]I-AB-MECA dissociation (B) binding assays with HEK293 cell membranes expressing Y2847.55C or Y2938.54F mutant receptors alone or in combination. The allosteric actions of LUF6000 were lost with either mutation, supporting the participation of π-π stacking with Y2847.55 and Y2938.54 and H-bonding between Y2938.54 and the exocyclic nitrogen of LUF6000 as critical interactions for LUF6000 binding to the proposed S4 allosteric site. For the [35S]GTPγS binding assays, concentration-response curves with Cl-IB-MECA were conducted in the presence of vehicle (DMSO, black) or 0.1 (yellow), 1 (blue), or 10 μM (magenta) LUF6000. Results were normalized to the Emax value of Cl-IB-MECA obtained in the presence of vehicle. The dotted line in each graph demarcates the Emax of Cl-IB-MECA in the presence of 10 μM LUF6000 with membranes from wild-type A3ARs (240 ± 11% of vehicle). EC50, Emax, and k values are reported in Supplemental Table 2. Data are presented as the mean ± S.D.