Figure 4.
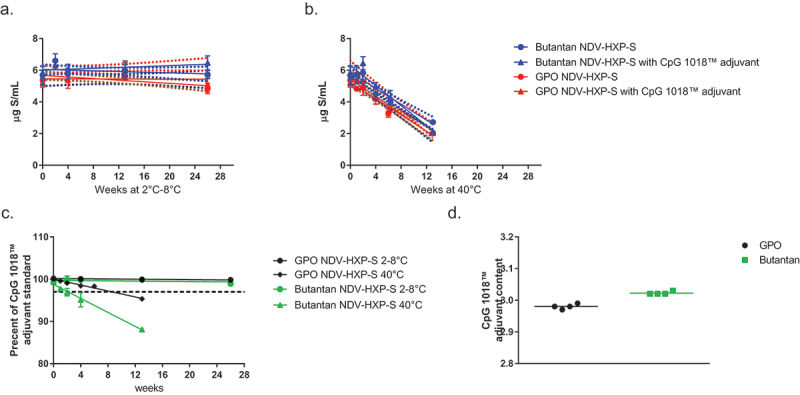
6-month stability of NDV-HXP-S with and without CpG 1018® adjuvant. NDV-HXP-S vaccine was prepared at 6 µg/mL S-antigen with and without 3 mg/mL CpG 1018® adjuvant in saline and held at 2°C to 8°C and 40°C. Samples were removed from temperature and tested for S-antigen content and stability by inhibition ELISA at selected timepoints. (a) NDV-HXP-S stability at 2°C to 8°C; (b) NDV-HXP-S stability at 40°C; (c) CpG 1018® adjuvant stability (integrity) at 2°C to 8°C and 40°C shown as a percentage of a CpG 1018® adjuvant standard tested at each time point; and (d) CpG 1018® adjuvant content at the beginning of the stability study. Methods provided by Dynavax technologies.
