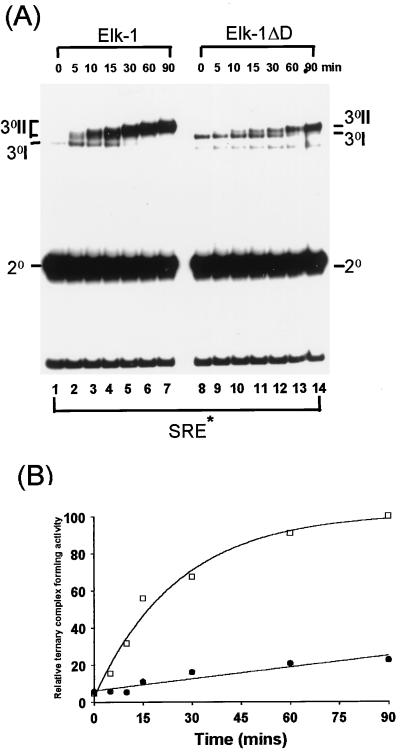
FIG. 3.
Deletion of the Elk-1 D domain perturbs the kinetics of ERK2 phosphorylation-induced ternary-complex formation. Elk-1 and Elk-1ΔD were phosphorylated in vitro by activated-ERK2 MAP kinase for the indicated times. (A) Kinetic study of ternary-complex formation of Elk-1 or Elk-1ΔD with SRF and a 134-bp fragment of the c-fos promoter containing the SRE (SRE*). The locations of the binary SRF-SRE complex (2°), unphosphorylated ternary complex (3°I), and multiple phosphorylated forms of ternary complex (3°II) are indicated. A band resulting from C-terminally truncated Elk-1ΔD runs below these ternary complexes. (B) The graph represents the quantification of the data shown in panel A. Data are presented relative to WT Elk-1 binding after 90 min (taken as 100). Open squares indicate wild-type Elk-1; solid circles represent Elk-1ΔD.