Abstract
Rationale:
Rapidly evolving e-cigarette technology developed for self-administering nicotine aerosol has the potential to be utilized to self-administer other aerosolized drugs of abuse. Rodent models which mirror characteristics of human e-cigarette use are necessary to explore the degree to which this may be a public health concern.
Objectives:
Our goal was to develop a highly translational model of discrete nose-only aerosol puff drug delivery to explore the reinforcing effects of fentanyl and sufentanil aerosols in rats.
Methods:
Male and female Sprague-Dawley rats were trained to perform a multiple schedule FR1 lever-press, 4s (second) nose hold operant during which the subject’s orofacial areas were exposed to drug-free glycerol/propylene glycol aerosol produced by a commercial e-cigarette at a power setting of 18 watts. Each completed 4s drug-free vehicle aerosol exposure resulted in a 3s presentation of a 0.1 ml dipper of sweetened milk solution. After training, rats were then allowed to self-administer 4s nose-only puffs of fentanyl (100–6000 ug/ml) or sufentanil (30–500 ug/ml) aerosol in the absence of paired milk dipper reinforcers.
Results:
All 31 rats learned the lever-press/nose poke multiple schedule for milk dippers alone and 25 accepted exposure to 4s of 18w of drug-free vehicle aerosol when paired with milk dipper presentations. In the absence of paired milk dipper presentations, fentanyl aerosol puffs at concentrations of 1000 and 3000 ug/ml as well as 100 ug/ml puffs of sufentanil served as reinforcers compared to both air puffs and drug-free vehicle aerosol puffs. There were no significant differences between males and females in number of fentanyl or sufentanil puffs self-administered.
Conclusions:
Discrete nose-only puffs of two potent opioids under exposure conditions comparable to puff durations in human e-cigarette users serve as reinforcers in rats. This outcome suggests that under appropriate conditions e-cigarettes might be a potential alternative delivery mechanism for illicit opioids.
Keywords: e-cigarette, vapor, fentanyl, sufentanil, reinforcement, self-administration
Introduction
Electronic cigarette use has skyrocketed over the past decade. The U.S. Centers for Disease Control estimates that e-cigarette use in high school students rose 900% between 2011 and 2015 and they are now more commonly used by youths in the United States than are conventional tobacco products (e-cigarettes.surgeongeneral.gov). Most e-cigarette users vape nicotine-containing e-juice, but current generation e-cigarettes have the potential to aerosolize any illicit drug which can be solubilized in a suitable vehicle. Inhalation drug delivery provides rapid CNS access and bypasses first-pass metabolism, therefore, the potential for abuse of aerosolized drugs using e-cigarette technology could be significant.
There are a number of additional factors which might modulate the relative abuse liability of drugs delivered as aerosols. The most important of these is likely to be potency. E-cigarette devices only aerosolize a small volume of liquid to generate each puff inhaled by the user. Nicotine is highly potent and newer more advanced e-cigarette devices appear to be capable of producing subjective ratings of drug satisfaction and nicotine blood levels near or equal to those resulting from smoked tobacco products (Hajek et al., 2017; St Helen et al., 2016; St.Helen et al., 2016). In contrast, drugs with insufficient potency to produce pharmacological effects after e-cigarette aerosol inhalation or those which require users to expend quantities of drug that could be more efficiently administered via other routes might be less likely to be abused using e-cigarettes. Unfortunately, some of the most concerning drugs of abuse at the current time are highly potent and relatively inexpensive synthetic opioids such as fentanyl and fentanyl analogs. In clinical studies, nebulized fentanyl aerosol produces similar analgesic efficacy as intravenous fentanyl (Thompson and Thompson, 2016). It is likely that other physiological effects of fentanyl such as rewarding effects can likewise be produced by aerosol delivery. There have been numerous highly publicized reports of deaths attributed to fentanyl delivered by e-cigarettes and multiple recent DEA notices have been published citing detection of fentanyl-adulterated e-juice in products marketed as nicotine-containing e-juices but the extent to which experimentation with aerosolized opioids is occurring is difficult to quantify.
Preclinical data on the abuse liability of opioid aerosols is limited. Three early studies conducted before the widespread availability of current generation high output e-cigarettes examined sufentanil aerosols generated by an ultrasonic nebulizer system (Jaffe et al., 1990, 1989; Weinhold et al., 1993). The authors found that rats would lever-press under a fixed-ratio 5 response schedule in 2-h daily sessions for sufentanil aerosol. Each 5s delivery of aerosolized sufentanil into an exposure chamber was followed by a 15s dwell period followed by a 60s fan-forced evacuation of spent aerosol from the chamber. Puffs of 25–75 ug/ml sufentanil exceeded puffs for the water vehicle, animals lost weight during the period of testing and pre-session administration of naloxone reduced the number of sufentanil puffs self-administered to levels similar to water aerosol suggests pharmacologically-relevant effects were produced. More recently, it was demonstrated that rats as well as mice will self-administer both fentanyl and sufentanil using e-cigarette based aerosolization systems and that drug exposure using these systems produces both measurable blood drug concentrations as well as physiologically relevant effects (Gutierrez et al., 2020; McConnell et al., 2021; Moussawi et al., 2020; Vendruscolo et al., 2018). When combined, the available preclinical data and anecdotal human reports suggest that aerosolized opioid delivery could become a significant public health concern. Additional data in animals using conditions which more closely mimic likely usage patterns in humans are necessary to further investigate this possibility.
One consistent feature of all prior preclinical rodent self-administration studies of which we are aware was the utilization of a full body exposure methodology in which operant responding resulted in the entire experimental chamber containing the animal being flooded with drug-laden aerosols (Frie et al., 2020; Gutierrez et al., 2020, 2021a, 2021b; Jaffe et al., 1989, 1990; Javadi-Paydar et al., 2019; McConnell et al., 2021; Nguyen et al., 2016a; Vendruscolo et al., 2018; Weinhold et al., 1993). This full-body exposure approach has both advantages and limitations. The chief advantages of this method are the relative simplicity of the apparatus and the ability to expose subjects to drug aerosols for extended durations. However, the physical limitation of the rapidity at which relatively large, whole body exposure chambers can be filled with aerosol and the length of time required to evacuate spent aerosol from the chamber present challenges in the ability to model human aerosol inhalation behavior. Specifically, human e-cigarette users typically inhale intermittent, short 3–5s puffs of aerosol (St.Helen et al., 2016; Voos et al., 2020). In intravenous self-administration studies it has been demonstrated that longer infusion durations reduce the reinforcing efficacy of a drug compared to shorter infusion durations (Balster and Schuster, 1973). Therefore it is possible that exposure durations in rodent experiments could influence the reinforcing efficacy of drug aerosols. A second concern with whole body exposure is that the animal as well as the entire test chamber environment is subject to progressively increasing condensed aerosol contamination after each puff. This could result in oral ingestion of drug by licking and grooming behavior innate to rodents and a secondary but physiologically-relevant route of drug exposure, especially given the high potencies of drugs such as synthetic opioids.
The primary goal of the present study was to develop and characterize a rodent model of e-cigarette delivered opioid aerosol reinforced behavior which recapitulates human use patterns as closely as possible to further elucidate the potential abuse liability of aerosolized opioids. To achieve this goal we designed and constructed a rodent e-cigarette aerosol self-administration system that restricted e-cigarette derived aerosol exposure to the orofacial region of the subjects. Rodents are obligate nose breathers, therefore we hypothesized this would produce primarily inhalational aerosol exposure and limit the possibility of oral drug ingestion. To facilitate acquisition of the aerosol delivery operant we utilized a paired liquid reinforcer under a multiple schedule procedure.
Materials and Methods
Subjects
Adult Sprague-Dawley rats (24 male/9 female) purchased from Charles River Laboratories (Frederick, MD) were used as subjects. Rats were at least 60 days of age prior to initiation of experiments. Rats were housed on a 12/12h reverse light/dark cycle (lights off at 0600) in clear polycarbonate microisolator cages with corncob bedding. Rats were tested between the hours of 0700–1300. Rats were fed a daily allotment of standard rodent chow (Harlan, Madison, WI) in quantities necessary to maintain healthy weights and prevent obesity. Water was available ad libitum except during experimental sessions. All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Compounds.
USP vegetable glycerin and propylene glycol were obtained from Fisher Scientific. Fentanyl hydrochloride and sufentanil citrate were obtained from the National Institute on Drug Abuse Drug Supply Program. E-liquid was prepared in the laboratory by combining 50% USP-grade propylene glycol and 50% USP-grade glycerin by volume (Fisher Scientific). The e-liquid was shaken well and then stirred on a magnetic bar stir-plate for a minimum of 12 hrs prior to use. Drug-containing e-liquids were prepared by weighing the appropriate amount of drug for a given concentration and then combining the powered drug with drug-free vehicle. The mixture was then stirred using a magnetic bar stir-plate for a minimum of 12 hrs to fully dissolve the drug into the vehicle solution. The milk solution used as a liquid reinforcer was prepared daily and consisted of a mixture of 25% powdered nonfat milk, 25% cane sugar and 50% tap water by volume.
Apparatus.
Aerosol self-administration training and testing was conducted in six modified rat operant conditioning chambers (Diagram 1) constructed of acrylic, stainless steel and aluminum walls with a stainless steel bar floor (Coulbourn Instruments, Allentown PA). The front wall of each chamber consisted of a slotted 3-section response panel. Two response levers were located 6 cm from the chamber floor in the right as well as the left slot of the chamber front wall. Above each response lever was a 3 watt (w) amber LED stimulus light. In the center slot of each front panel, 6 cm from the floor, was a 3.5 cm circular aperture into which the rat could insert their head. Attached behind the aperture was a custom manufactured acrylonitrile butadiene styrene (ABS) plastic aerosol delivery apparatus. Plastic components of the apparatus were designed using Autodesk Fusion 360 computer-aided 3-dimensional modeling software (San Rafael, CA) and printed by fused deposition modeling on a Flashforge Creator Pro 3D printer (Jinhua City, Zhejiang, China). The apparatus consisted of a 40×40 mm square aerosol nose-poke exposure chamber with a multiport aerosol diffusion manifold incorporated into the upper rear quadrant of the chamber. The bottom of the nose-poke chamber contained a 10mm hole into which a 0.1 ml dipper cup could be elevated into the nose-poke chamber using an electrically-operated liquid dipper (Model ENV-202-M-S, Med Associates, Fairfax, VT). On the exterior side walls of the nose-poke chamber, 15 mm above the nose-poke chamber floor an infrared photobeam sensor emitter and receiver set was mounted to detect head entries (Adafruit Industries, New York, NY). A 3w amber stimulus lamp was located in the rear of the nose-poke chamber, opposite the head entry aperture. The top of the nose-poke chamber contained a 23 mm circular opening to which attached a 90 cm tall curved plastic exhaust chimney. A 25mm 12v variable speed axial muffin fan (Newark Electronics) was mounted at the upper end of the chimney in order to draw spent aerosol from the nose-poke chamber which was then exhausted into a fume hood. A 3w incandescent chamber houselight was located in the upper center of the rear wall of the operant chamber.
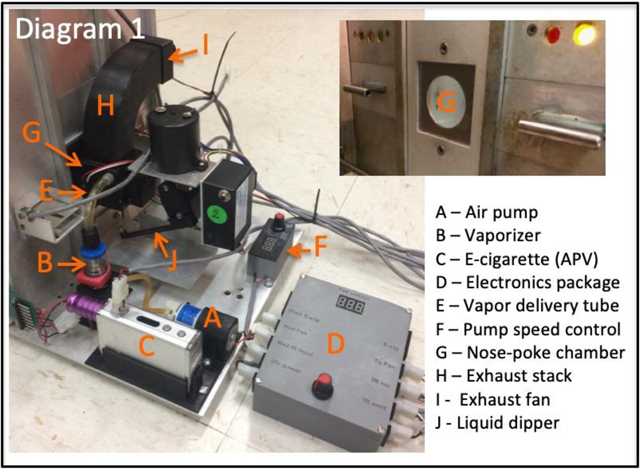
Aerosol was generated by an Innokin iSub 0.5 ohm stainless steel coil inserted into an Innokin iSubV Vape reservoir tank. The tank was attached to a Smoktek R200 200W Box Mod e-cigarette. The coil, reservoir and e-cigarette were purchased from a commercial source (Directvapor.com). The reservoir tank was modified by sealing the air intake slots at the base of the reservoir with epoxy and drilling and tapping the base of the tank to accept a 1/8 inch threaded hose barb. A length of latex tubing connected the hose barb to a computer-actuated variable speed 12v diaphragm air pump (American Science and Surplus, Chicago, IL). The aerosol outflow from the mouthpiece of the e-cigarette was directed by PVC tubing into a fitting molded into the outside of the diffusion manifold within the nose-poke chamber. The e-cigarette was modified to be triggered by a remote relay switch by partially disassembling the device to remove the tactile firing button, soldering two contact wires in place of the button contacts and reassembling the e-cigarette. The e-cigarette, photobeam detector, air pump and exhaust fan were connected to a commercial operant behavior interface, control and data acquisition system (Med Associates, Fairfax, VT) through the use of an in-house custom-designed relay and voltage conversion control board (Diagram 1).
System operation:
E-cigarette aerosol puffs were generated by the application of positive pressure to the e-cigarette reservoir tank by means of the air pump. The air pump output was adjusted to a rate of 1 liter/min by altering the driving voltage and the flow rate was verified by a high precision flowmeter (Matheson Tri-Gas, Montgomeryville, PA). Upon completion of the fixed-ratio (FR) lever-press requirement, the houselight was extinguished and the cue lamp within the nose-poke chamber was activated. Subsequent detection by the photobeam of the subject’s nose within the nose-poke chamber triggered a series of timed signals to each component. First, the e-cigarette heating coil was preheated for 1s. The air pump was then activated, pressurizing the e-cigarette reservoir tank and sending a stream of aerosol through the attached tubing and diffusion manifold into the nose-poke chamber. One second after the air pump was activated, the exhaust fan in the chimney of the apparatus was also energized, drawing spent aerosol from the nose-poke chamber at a rate set to balance input and maintain the visible aerosol cloud entirely within the nose-poke chamber. At the conclusion of the puff, the e-cigarette coil, air pump and nose-poke chamber cue lamp were deenergized. The fan remained activated for an additional 4s to clear all spent aerosol from the nose-poke chamber. In training sessions, the liquid dipper was elevated into the nose-poke chamber for a period of 3s after the completion of the aerosol puff, provided that the subject retained head position within the chamber for the entire duration of the puff as determined by the continuous interruption of the infrared photodetector within the chamber.
Aerosolization volume determination:
Prior to initiating rodent studies, an experiment was first conducted to characterize the relationship between e-cigarette output wattage setting and volume of e-liquid aerosolized using our system. An assay of a variety of commercially-produced e-liquids found that almost all contained primarily propylene glycol and vegetable glycerol as vehicles, often with a smaller percentage of water (EL-Hellani et al., 2018). For all of our studies we utilized a 50% propylene glycol and 50% vegetable glycerol to be consistent with this finding. For each determination, an e-cigarette reservoir tank was fitted with a fresh 0.5 ohm heating coil and filled with 3 ml of 50% propylene glycol/50% vegetable glycerol vaping solution. The filled reservoir tank was weighed on an analytical balance and then connected to the e-cigarette electrical circuit. The e-cigarette was actuated remotely for a total of 10, 4s puffs with a 20s timeout period between each puff. Positive-pressure air flow rate through the e-cigarette was set at 1 liter/min using a high precision flowmeter as described previously. The reservoir tank was then removed from the e-cigarette and reweighed to determine the total weight lost in mg. The specific gravity of the vehicle was used to convert the weight in mg to volume in ul. Total volume was divided by 10 to estimate the volume aerosolized per puff. This process was completed at e-cigarette wattage settings ranging between 5 and 18 watts with 5 replicates per wattage setting.
Puff training:
Naïve rats were first trained in daily 30-min operant sessions to insert their nose and forward portion of their head into the nose-poke chamber and break the photobeam, resulting in a 3s presentation of a 0.1 ml dipper cup containing sweetened milk solution. After subjects performed the head entry operant for milk dippers, the schedule was converted to a multiple schedule of lever-pressing and head entry. The subjects were first required to emit a FR1 response on the right lever within the operant chamber. Completion of the FR1 extinguished the operant chamber houselight and right lever light and illuminated the stimulus light within the nose-poke chamber. The subject was then required to place their head within the nose-poke chamber and break the photobeam for at least 0.5s, upon which the milk dipper elevated for 3s. The head entry hold time required was then increased across successive training days until 4s of uninterrupted head positioning within the nose-poke chamber was required for each 3s milk dipper presentation. Any head withdrawal before the specified time elapsed reset the schedule and stimulus lights back to the FR lever-press requirement and did not produce milk dipper presentation. One male subject could not be trained to maintain head position for 4 sec for milk dippers and did not advance beyond this point in training. Upon successful training of the multiple schedule lever-press/head entry operant, training progressed to include exposure to 4s of vehicle aerosol. Prior preliminary studies showed that aerosol-naïve rats would avoid exposure to vehicle vapor at high e-cigarette wattages, therefore, in the present study the e-cigarette output wattage was increased slowly over successive training sessions to 18 w. Each wattage was tested for a minimum of 5 sessions in a given subject or until responding stabilized as evidenced by no increasing or decreasing trends in responding in the last 3 sessions. This procedure was repeated over several progressive steps until 18w was achieved.
Test procedure:
Those rats that responded for greater than 20, 4s puffs of 18w of vehicle aerosol paired with 3s milk dipper access in 30 min advanced to the drug aerosol test phase of the study. Five male subjects failed to reach this criteria and did not advance to drug aerosol testing. One female developed mammary tumors and was euthanized prior to completion of testing. Drug aerosol tests were conducted in repeated 5-session blocks (M-F). Between testing of each drug concentration, vehicle aerosol paired with milk dipper presentation was available for at least 5 sessions or until responding stabilized as evidenced by no increasing or decreasing trends in the prior 3 sessions. This was followed by a 5-session block in which drug-containing e-liquid was substituted for drug-free e-liquid (vehicle aerosol) and dipper presentations were omitted (i.e. subjects responded for aerosol only). After completion of testing of a given drug concentration, the subjects returned to the vehicle aerosol+milk condition for at least 5 sessions and the process was repeated for each additional drug concentration examined. Fentanyl-containing e-liquid at concentrations of 100, 300, 1000, 3000 and 6000 ug/ml as well as fentanyl-free vehicle and an air-puff only aerosol-free extinction control were examined in a pseudo-random order balanced across subjects. After completion of the fentanyl concentration-effect curve, an identical procedure was used to examine sufentanil (30, 100 and 500 ug/ml) as well as sufentanil-free vehicle and an air-puff only aerosol-free extinction control.
Data Analysis:
E-cigarette aerosol output volume produced by 10 puffs was determined by converting the weight loss from the vaporizer into a volume in ul. The resulting volume was divided by 10 to estimate volume per puff. Five replicate determinations were performed at each wattage and mean values (+/− SEM) were calculated from this data. For self-administration studies, total daily session right and left lever presses and number of completed puffs for each subject were collected using MedPC V4 software (Med Associates, St. Albans VT, USA). Puffs were only registered as complete and recorded if the subject retained head position within the nose-poke chamber for the entire duration of the aerosol puff, which was 4 sec at the completion of drug-free paired dipper + puff training. Puffs of less than the programmed duration reset the sequence without being recorded as a completed puff. For each subject, a mean number of puffs completed was calculated based on the last 3 sessions at each test condition. The number of puffs for both fentanyl and sufentanil and inactive-lever responses were then analyzed according to a 2-step process using Prism 9 for Macintosh. The threshold for statistical significance for main effects and/or interactions was set at p< 0.05. Initially puffs were compared using a 2-way (animal sex X drug concentration) mixed-model analysis of variance (ANOVA). For both drugs, the main effect of sex and the interaction of sex x drug concentration were not statistically significant, therefore the data for each drug was collapsed across sexes and one-way within subject ANOVAs were conducted on total number of puffs received. Significant main effects of drug concentration were followed by Fisher’s post-hoc tests to determine those drug concentrations that differed from the no-puff extinction condition as well as the vehicle vapor condition. An identical 2-step statistical analysis process was completed on inactive-lever presses in the fentanyl and sufentanil experiment.
Results
Figure 1 shows aerosolization volume of 50% propylene glycol/50% vegetable glycerol vehicle as a function of increasing e-cigarette vaporizer output wattage. Data shown in filled squares are the means (+/− SEM) of 5 aerosolization volume determinations at each wattage setting. Individual determinations are shown in open circles. As e-cigarette wattage increased, aerosol volume per puff also increased. There was no detectable volume of vehicle solution aerosolized at an e-cigarette output of 5 watts. Detectable volume loss of 0.43 ul (+/− 0.08 ul) per puff occurred at an output of 9 watts. At 18 watts, the power setting utilized in the self-administration experiments with fentanyl and sufentanil, each 4s puff aerosolized a mean of 4.8 ul (+/− 0.19 ul) of vehicle vaping solution.
Fig 1.
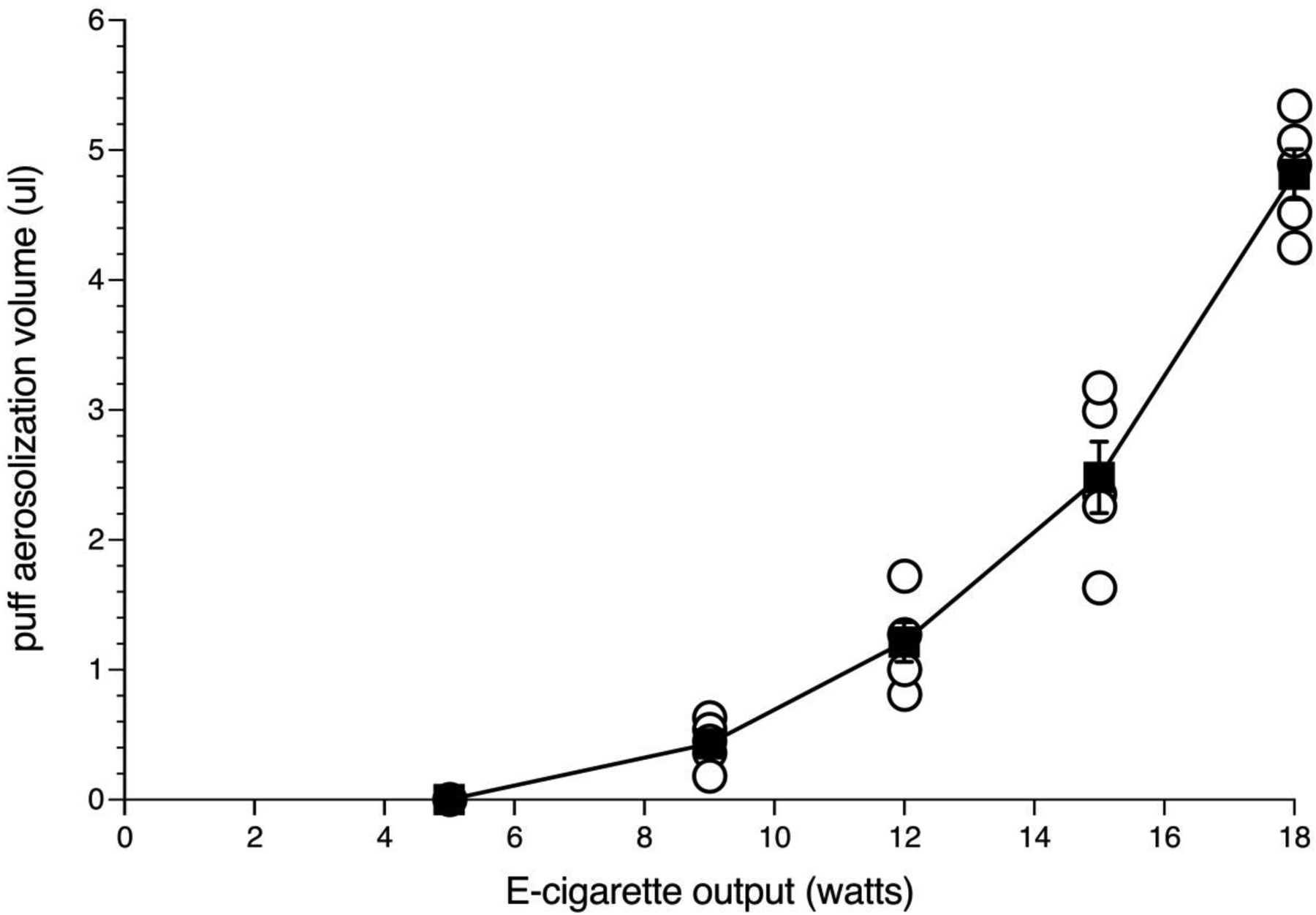
Effect of increasing e-cigarette output wattage setting on total aerosolization volume of 50% propylene glycol/50% vegetable glycerol vehicle. Mean (+/− SEM) vehicle volume (ul) aerosolized per puff is shown by filled squares. Open circles show volume aerosolized in each of the 5 replicate determinations at each wattage setting.
Figure 2 shows results from male (n=14) and female (n=6) rats examining the effect of systematically increasing the wattage which increased vehicle aerosol exposure density present in the nose-poke chamber on total number of 4s aerosol puffs completed when each completed puff was reinforced by milk dippers. At the 0 watt condition, males earned a mean of 60 paired puffs+dippers and females earned a mean of 82 puffs+dippers. At 18 watts, males earned a mean of 73 paired puffs+dippers and females earned a mean of 76 puffs/dippers. There was no main effect of e-cigarette wattage on puffs+dippers [F(6,120)=0.900, p=0.497]. There was also no main effect of sex [F(1,20)=0.873, p=0.361] on puffs+dippers. The interaction of e-cigarette wattage x sex also failed to reach statistical significance [F(6,120)=2.092, p=0.059]. It required a mean of 46 experimental sessions (+/− 2.5) to reach the final drug-free training condition of 4s head entry/18w vehicle aerosol + 3s milk dipper training condition prior to initiating opioid exposure testing. The number of sessions required to reach the 4s hold at each increase in e-cigarette output wattage was highly subject-dependent with some subjects quickly becoming acclimated to higher wattages, while others required several days over which behavior was initially suppressed before rebounding and stabilizing.
Fig 2.
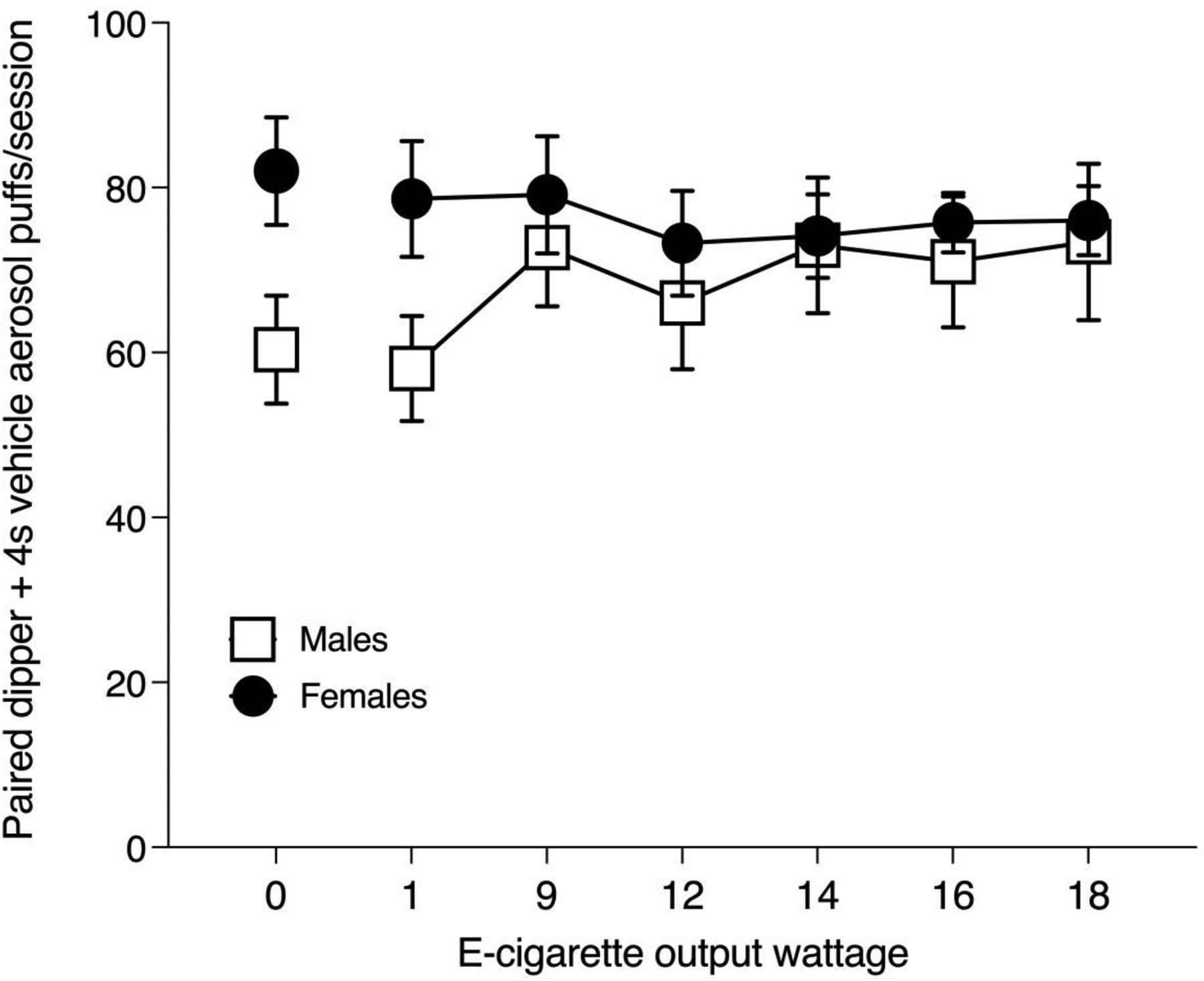
Effect of increasing e-cigarette output wattage during training on number of 4s nose-only puffs of 50% propylene glycol/50% vegetable glycerol vehicle aerosol received when reinforced by 3s of access to a 0.01 ml liquid dipper containing sweetened milk. Mean (+/− SEM) aerosol vehicle vapor puffs per 30-min test session received by male rats are shown in open squares (◻) and females are shown in filled circles (●).
The reinforcing effects of fentanyl aerosol puffs were examined in 14 male and 6 female rats. Mean aerosol puffs per session for concentrations of 100 to 6000 ug/ml fentanyl, in the absence milk dipper presentations as well as an air puff-only extinction condition and the vehicle aerosol condition are shown in the upper panel of figure 3. Fentanyl aerosol puff self-administration produced an inverted U-shaped concentration-effect curve reaching a maximum of 17 puffs/30-min session at a concentration of 3000 ug/ml. A two-way ANOVA (concentration x sex) revealed a significant effect of fentanyl concentration [F(6,108)=3.755, p=0.002] but no main effect of sex [F(1,18)=1.146 p=0.299] nor a concentration x sex interaction [F(6,108)=1.668, p= 0.136], therefore the data from males and females were combined, plotted together and reanalyzed by 1-way ANOVA. A 1-way ANOVA demonstrated a significant main effect of fentanyl concentration [F(6,114)=3.939, p=0.001]. Post-hoc Fisher tests revealed that puffs of 300–6000 ug/ml fentanyl were self-administered in significantly (p<0.05) greater numbers than air puffs and that puffs of 1000 and 3000 ug/kg fentanyl were self-administered in significantly greater numbers than drug-free vehicle puffs. There was no significant difference between the number of air puffs obtained and number of drug-free vehicle puffs obtained. The lower panel of figure 3 shows inactive-lever responding under vehicle control conditions and each fentanyl aerosol puff test concentration. Inactive-lever pressing was uniformly low, peaking at 3.4 responses per 30-min session at the 1000 ug/ml fentanyl puff condition. There was no main effect of fentanyl aerosol puff concentration on inactive-lever responding (F[6,114]=0.684, p=0.663).
Fig 3.
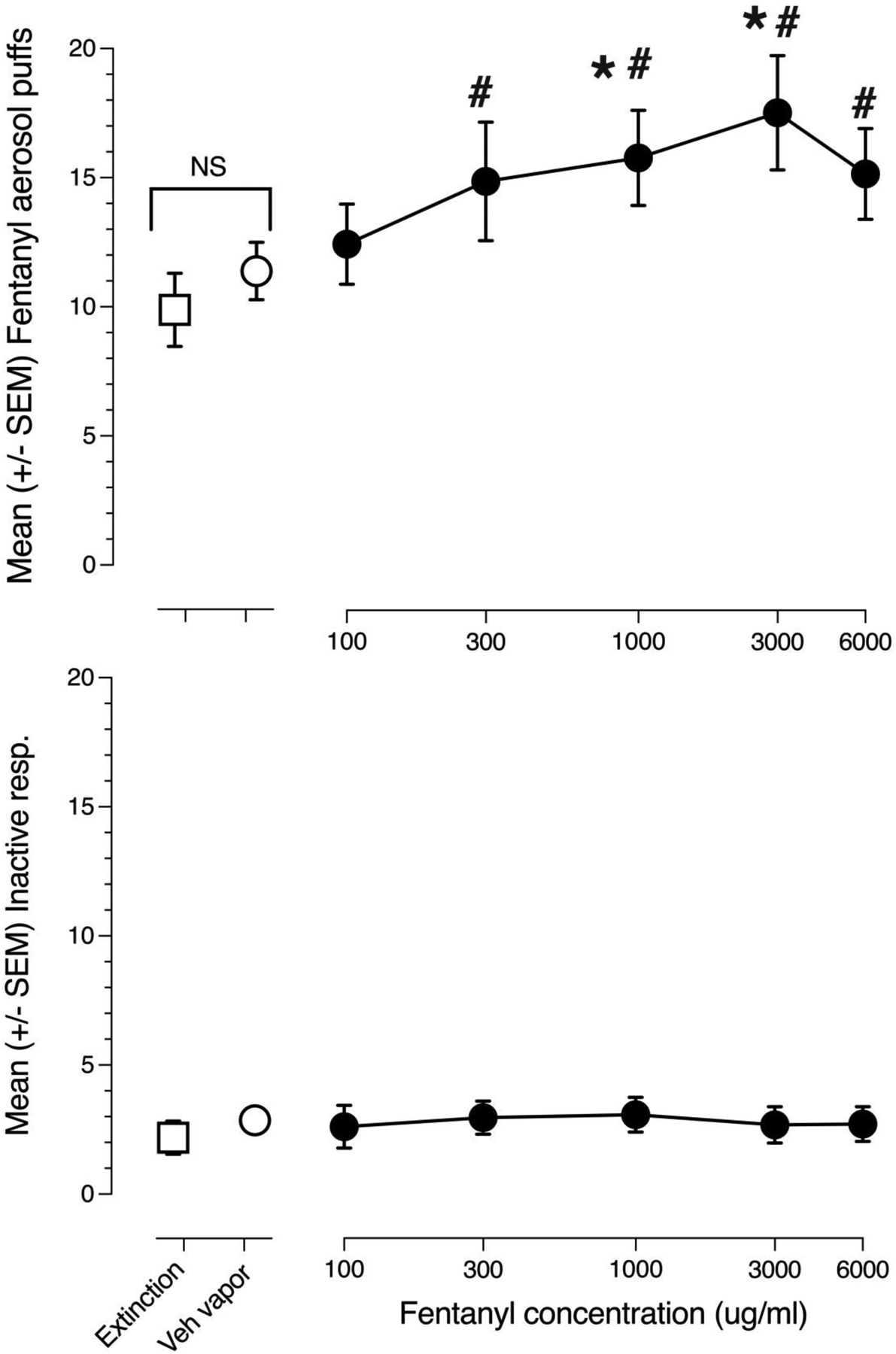
Concentration effect curves for fentanyl aerosol puff self-administration (n=20). The upper panel shows mean (+/− SEM) 4s/18 watt fentanyl aerosol puffs obtained in the last three 30-min test sessions at each of the fentanyl liquid concentrations (●). Also shown are the mean (+/− SEM) air only puffs obtained (◻) and mean (+/− SEM) puffs of fentanyl-free, 50%vegetable glycerol/50% propylene glycol vehicle (○). The lower panel shows mean (+/− SEM) inactive-lever responses emitted in the last three 30-min test sessions at each fentanyl concentration (●). Also shown are the mean (+/− SEM) inactive-lever responses emitted in the air puff test condition (◻) and mean (+/− SEM) inactive-lever responses in the fentanyl-free vehicle test condition (○). # indicate statistically significant (p< 0.05) differences compared to air puffs. * indicate statistically significant (p< 0.05) differences compared to drug-free vehicle aerosol. Bracketed NS indicates no statistically significant difference between air puffs and drug-free vehicle aerosol puffs.
The reinforcing effects of sufentanil aerosol puffs were examined in 11 male and 7 female rats. Of these, 2 males and 2 females were drug naïve while 9 males and 5 females had been used in the prior tests with fentanyl. A two-way ANOVA (sufentanil concentration x sex) revealed a significant main effect of sufentanil concentration [F(4,64)=3.281, p=0.017] but no main effect of sex [F(1,16)=0.021, p=0.887] nor a concentration x sex interaction [F(4,64)=1.353, p=0.298] therefore the data from both sexes was pooled and reanalyzed by 1-way ANOVA. Figure 4 shows sufentanil aerosol puffs at concentration of 30, 100 and 500 ug/ml as well as air only puffs and drug-free vehicle aerosol puffs. Sufentanil produced an inverted-U shaped concentration-effect curve with a mean of 18 puffs/30-min session at the intermediate 100 ug/ml sufentanil concentration (upper panel). There was a main effect of sufentanil concentration on number of puffs administered [F(4,68)=3.161, p=0.019]. Post-hoc Fisher tests revealed that the 100 ug/ml sufentanil concentration resulted in a significantly greater number of puffs compared to both the air puffs as well as drug-free vehicle. The lower panel of figure 4 shows inactive-lever responding during extinction, the drug-free vehicle control condition and each sufentanil aerosol puff test concentration. Inactive-lever pressing was uniformly low with a mean of less than 3 responses/session across all tests. There was no main effect of sufentanil concentration on inactive-lever responding (F[4,68]=0.102, p=0.981).
Fig 4.
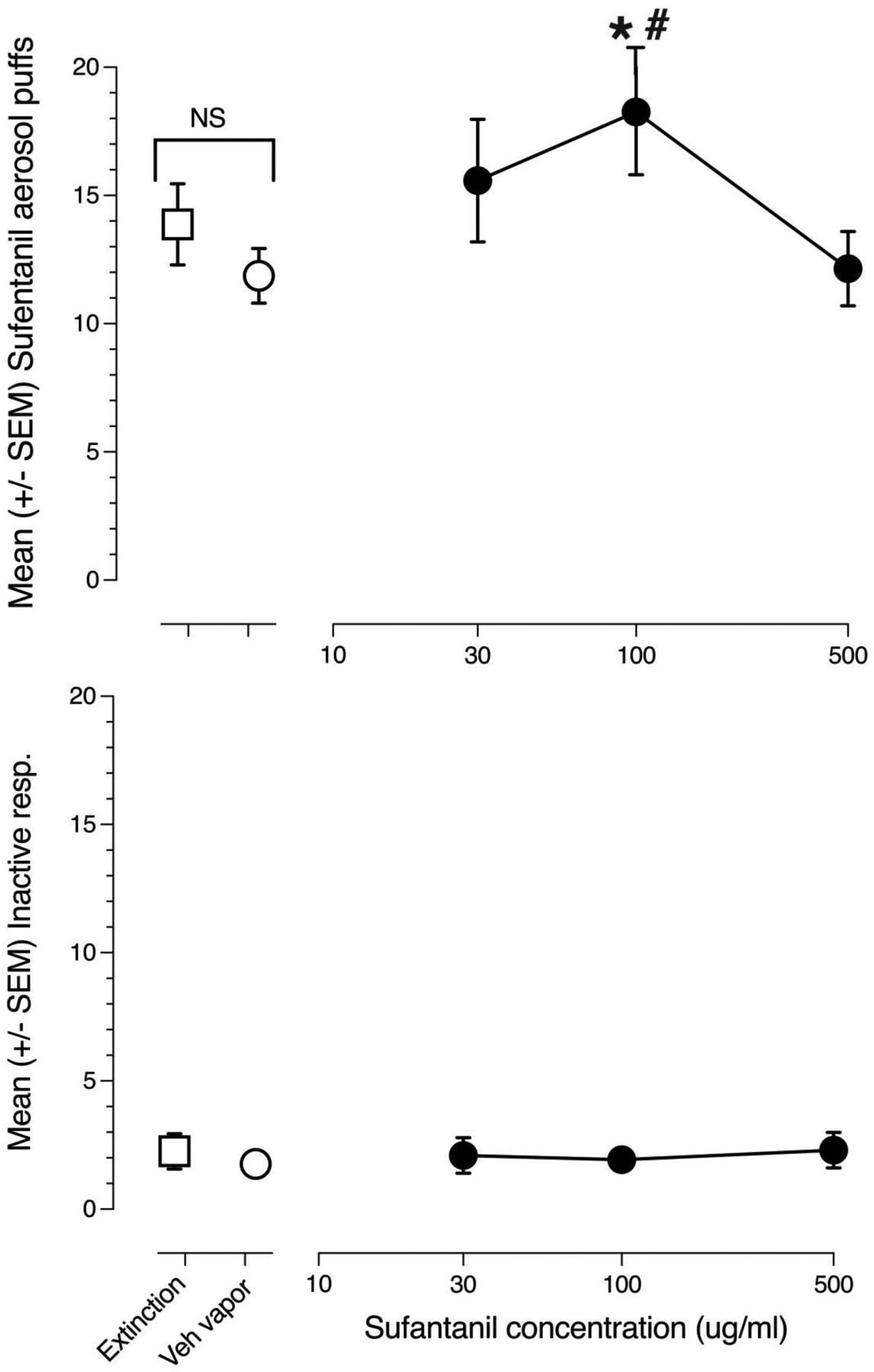
Concentration effect curves for sufentanil aerosol puff self-administration (n=18). The upper panel shows mean (+/− SEM) 4s/18 watt sufentanil aerosol puffs obtained in the last three 30-min test sessions at each of the sufentanil liquid concentrations (●). Also shown are the mean (+/− SEM) air-only puffs (◻) and mean (+/− SEM) puffs of sufentanil-free vehicle (○). The lower panel shows mean (+/− SEM) inactive-lever responses emitted in the last three 30-min test sessions at each sufentanil concentration (●). Also show are the mean (+/− SEM) inactive-lever responses emitted in the air puff test condition (◻) and mean (+/− SEM) inactive-lever responses in the sufentanil-free, 50% vegetable glycerol/50% propylene glycol vehicle control condition (○). # indicate statistically significant (p< 0.05) differences compared to air puffs. * indicate statistically significant (p< 0.05) differences compared to drug-free vehicle aerosol. Bracketed NS indicates no statistically significant difference between air puffs and drug-free vehicle aerosol puffs.
Discussion
The present data demonstrate that rats can be trained to self-expose to 18w/4s puffs of drug-free e-cigarette aerosol when food delivery is made contingent upon aerosol exposure. The e-cigarette power setting of 18w produced a thick aerosol cloud in the nose-poke chamber that rats initially avoided. By gradually increasing the e-cigarette wattage, the total number of vehicle aerosol puffs accepted could be maintained at levels no different from the aerosol-free air-puff initiation condition. There was a trend toward an interaction of sex and e-cigarette wattage in which responding in the female rats was greater than the male rats at the 0 and 1 watt e-cigarette output settings. The study utilized a greater number of males than females, therefore, the failure to reach statistical significance is probably the result of insufficient statistical power to resolve sex differences. For the e-cigarettes used in the present study, wattages below 9 watts did not produce measurable aerosol generation (see figure 1). At e-cigarette wattages of 9 watts and higher, which did generate aerosol, the data from males and females converged (figure 2). As such it is more likely that the potential sex difference is related to a difference in milk reinforcer training rather than a result of aerosol exposure itself.
In the absence of paired milk dipper deliveries, 4s nose-only puffs of both fentanyl and sufentanil aerosol served as reinforcers in rats, significantly exceeding puffs of vehicle aerosol alone as well as an air-puff only aerosol-extinction test condition. Puffs of fentanyl and sufentanil both produced inverted U-shaped concentration-response curves with a similar number of puffs self-administered per 30-min session at optimal concentrations of each drug. The number of puffs of drug aerosol self-administered in the absence of milk reinforcers was considerably lower than the number of puffs of vehicle aerosol when combined with milk dippers. This is likely due to differences across reinforcer classes as it is not uncommon for rats to achieve very high numbers of highly palatable food reinforcers earned per experimental session, whereas drug reinforcer self-administration is impacted by the direct pharmacological effects of the drug, resulting in titration of responding. Regardless, the number of opioid aerosol puffs self-administered in the present study was as great or greater than the number of opioid aerosol puffs self-administered in previously reported studies, especially when scaled for session duration which varied widely across experiments (Gutierrez et al., 2020; Jaffe et al., 1990, 1989; Moussawi et al., 2020; Vendruscolo et al., 2018; Weinhold et al., 1993). The number of fentanyl vapor puffs per unit time in the present study also are well within the range of those reported in rats self-administering fentanyl intravenously when adjusted for session duration (Malone et al., 2021; Reiner et al., 2021; Sustkova-Fiserova et al., 2020). These results extend and complement a growing body of data from previous full body opioid exposure studies showing that rats will self-administer puffs of sufentanil aerosols generated by an ultrasonic neubulizer as well as fentanyl, sufentanil and heroin aerosols generated by e-cigarettes (Joan W. Flacke et al., 1985; Gutierrez et al., 2020; Jaffe et al., 1989, 1989; McConnell et al., 2021; Moussawi et al., 2020; Vendruscolo et al., 2018; Weinhold et al., 1993).
Aerosol inhalational self-administration in rodents has been proposed as a compliment or even an alternative to the more traditional route of intravenous self-administration (Frie et al., 2020; Gutierrez et al., 2020, 2021a; Javadi-Paydar et al., 2019; McConnell et al., 2021; Miliano et al., 2020; Moussawi et al., 2020; Nguyen et al., 2016a; Vendruscolo et al., 2018). Prior experiments in rodents have demonstrated that opioids, psychomotor stimulants, nicotine and cannabinoid aerosols produce many of the same physiological and neurochemical responses as when given by other more traditional routes (Gutierrez et al., 2021a, 2020; Harris et al., 2018; Javadi-Paydar et al., 2018; Nguyen et al., 2016a, 2016b; Taffe et al., 2021). As such, hypothesizing inhalational self-administration as an alternative to intravenous self-administration has merits. However there are a number of unique aspects associated with inhalational drug delivery and technical challenges which must be addressed if the method is to be accepted and more widely adopted.
One question to be addressed is the degree to which inhalation drug self-administration is governed exclusively by the pharmacological effects of the drug or whether other non-pharmacological factors may also be important in controlling the behavior. Prior studies have shown that both fentanyl and sufentanil aerosol exposure produce physiological effects, supporting the hypothesis that the pharmacological effects of self-administered fentanyl and sufentanil aerosol are behaviorally relevant (Gutierrez et al., 2021a; Jaffe et al., 1989; Moussawi et al., 2020; Vendruscolo et al., 2018). An additional line of evidence to support this hypothesis would be to demonstrate that the relative potency of fentanyl and sufentanil in the aerosol self-administration procedure are comparable to the relative potencies of the two drugs when administered by other routes and in different types of behavioral procedures. We are unaware of any studies that have directly compared intravenous self-administration of sufentanil and fentanyl in rats in the same experiment. However, in assays of antinociception and respiratory suppression in rodents, sufentanil is approximately 10- to 30-fold more potent than fentanyl (J. W. Flacke et al., 1985; van den Hoogen and Colpaert, 1987; Yeadon and Kitchen, 1990). In the present study both fentanyl and sufentanil produced a similar peak number of puffs self-administered but sufentanil was approximately 30-fold more potent than fentanyl. Therefore our self-administration data is consistent with established potency ratios between the two drugs.
Another important question is the extent to which aerosol self-administration studies using differing methods produce comparable data. There are numerous technical differences across published studies which make comparisons challenging. Studies examining e-cigarettes as nicotine delivery devices have demonstrated that e-cigarette power settings, air flow rates, aerosolization vehicle composition and other factors impact the amount of nicotine aerosol generated by the devices (DeVito and Krishnan-Sarin, 2018; Kosmider et al., 2018) as well as influence inhalable particle size which has implications for drug absorption (Mulder et al., 2019). These factors would be expected to have similar effects upon the aerosolization of opioids. Even were one to assume that aerosolization device characteristics were not a significant concern, other methodological factors such as duration of exposure, which is known to impact the abuse-related effects of inhaled drugs (Shelton and Slavova-Hernandez, 2009) have varied widely across experiments.
In the present experiment, peak numbers of 4s fentanyl puffs occurred when subjects were allowed to self-administer fentanyl aerosol generated from a 3 mg/ml solution. A prior experiment conducted in rats utilized a fixed concentration of 10 mg/ml fentanyl (McConnell et al., 2021) while a study in mice noted peak self-administration rates when using a 2.5 mg/ml fentanyl solution (Moussawi et al., 2020). These concentration are remarkably similar even though there were many other dissimilarities in exposure parameters such as e-cigarette type, output power and aerosol contact time. This might suggest that a fairly narrow range of opioid e-liquid concentrations will support aerosol self-administration in rodents, and other methodological differences may have less impact. This hypothesis is weaker when comparing reported sufentanil liquid concentrations across aerosol experiments. The optimal self-administration concentration of 100 ug/ml sufentanil in the present study is similar to that from three prior experiments reporting peak self-administration at nebulized sufentanil liquid concentrations of 50–75 ug/ml (Jaffe et al., 1990, 1989; Weinhold et al., 1993). This was despite the puff duration being 4s in the present study and 15s in the earlier experiments. However, in a more recent report, a dramatically lower concentration of 1.65 ug/ml sufentanil, at a puff duration of 10s supported self-administration, while 10 ug/ml sufentanil resulted in fewer aerosol deliveries (Vendruscolo et al., 2018). Additional data across a more extensive and systematically manipulated series of conditions will be necessary to fully address the relationship between aerosolization liquid drug concentration, puff durations and nose-only vs full body exposure kinetics in order to determine the extent to which they might impact relevant endpoints.
Another significant complicating factor for studies examining drugs self-administered as aerosols are the potential effects exerted by the vehicle itself. In intravenous self-administration experiments, the drug vehicle is typically saline which has little physiological impact or reinforcing efficacy, therefore, a greater number of drug than vehicle infusions are typical even at low work requirements such as FR1 (Awasaki et al., 1997; Sharp et al., 2021). The earliest reports of sufentanil aerosol self-administration used a water-based vehicle with an ultrasonic nebulizer and found that although response rates for sufentanil were low, operant responding for drug-containing aerosol exceeded that of water aerosol (Jaffe et al., 1990, 1990; Weinhold et al., 1993). In contrast, the vehicles utilized for e-cigarette based aerosolization typically contain propylene glycol and vegetable glycerol either alone, or more commonly in some proportional mixture which varies widely across products (Li et al., 2020). Human e-cigarette users describe vegetable glycerol as having a sweet flavor while propylene glycol is utilized to provide a “throat hit” reminiscent of traditional cigarettes (Harvanko et al., 2019; Rao et al., 2018). The degree to which the vehicle itself impacts drug aerosol self-administration in rodent studies is unclear. One experiment in mice found that that puffs of vehicle aerosol self-administered exceeded puffs of 2.5, 5 and 10 mg/ml fentanyl aerosol self-administered under a FR1 schedule (Moussawi et al., 2020). In a prior study in rats, active nose pokes for sufentanil aerosol presentation were lower than nose pokes for vehicle vapor + drug paired cues, vehicle vapor without cues, cues without vapor or a no vapor or cue extinction condition (Vendruscolo et al., 2018). Female mice responding for heroin aerosol using a FR1 lever-press without a nose-poke requirement only showed significantly higher drug-lever vs vehicle-lever responding on three non-consecutive days of a 10 day acquisition period (Gutierrez et al., 2021a). In the present experiment, active-lever responding for vehicle aerosol as well as active-lever responding during air-puff only extinction tests exceeded inactive-lever responding which can likely be attributed to conditioned reinforcing effects resulting from the continued response-contingent presentation of visual stimulus light cues and air pump activations which had also been paired with drug aerosol delivery. However, propylene glycol/vegetable glycerol vehicle aerosol puffs were almost identical to air only puffs suggesting a lack of primary reinforcing effects of vehicle aerosol. Importantly, in contrast to prior studies, both fentanyl and sufentanil produced a statistically greater number of active-lever responses and drug aerosol puffs than did drug-free vehicle aerosol or air puffs. Further, at least one concentration of drug aerosol was self-administered at levels significantly greater than drug-free vehicle by 17 of 20 subjects in the fentanyl experiment and 15 of 18 subjects in the sufentanil experiment. Taken together these data clearly demonstrate that both fentanyl and sufentanil aerosol were serving as positive reinforcers.
One hypothesis for why the present procedure resulted in fentanyl and sufentanil aerosol demonstrating reinforcing effects whereas the drug-free vehicle did not may be the complex multiple schedule of lever-press+nose pokes we employed. The paired vehicle aerosol+milk dipper procedure utilized to promote consistent 4s nose-only aerosol exposures during acquisition could have also played a role. Increasing work requirements or using complex schedules as a means to differentiate the relative reinforcing efficacy of drugs from one another have been used previously in a variety of contexts. For instance, in primates, combinations of methadone and ethanol or methadone and cocaine were only demonstrated as being more reinforcing then either drug alone when the fixed-ratio value was high (Shelton et al., 1998; Wang et al., 2001). Similarly, in rats, combinations of intravenously self-administered heroin combined with cocaine maintained responding greater than vehicle or either drug alone at a higher fixed-ratio value (Martin et al., 2006). Progressive ratio schedules and choice procedures have likewise been effective in differentiating the relative reinforcing effects between two drugs and between drugs and alternative non-drug reinforcers (Gauvin et al., 2018; Panlilio et al., 2017; Richardson and Roberts, 1996). The possibility that the higher work requirement of the multiple schedule was a factor is also consistent with the observation from a prior study where mice defended their exposure to fentanyl puffs more strongly than vehicle aerosol puffs at FR10 compared to FR1 (Moussawi et al., 2020). The possibility that the milk dipper-reinforced vehicle aerosol acquisition procedure itself was responsible for promoting sufficient drug exposure to facilitate the acquisition of fentanyl and sufentanil aerosol-reinforced behavior will require additional data to address. Specifically it will be necessary to assess if rats will self-administer nose only opioid aerosol puffs in the absence of any prior training with another reinforcer. Although that is possible, we speculate that the complex, multiple schedule of lever pressing and extended nose hold utilized in the present study to mimic puff durations typical of human e-cigarette users (St.Helen et al., 2016; Voos et al., 2020) is unlikely to be acquired by rats without considerable training.
In summary, the present data demonstrate that discrete 4s nose-only puffs of both fentanyl and sufentanil aerosol delivered by a standard commercial e-cigarette will be self-administered by rats. Under a multiple schedule, which imposes relatively stringent response costs, both fentanyl and sufentanil aerosol reinforced lever-pressing, whereas the propylene glycol/vegetable glycerol vehicle did not. Although it was not a focus of the present experiment, there does not appear to be any sex differences in self-administration of either fentanyl or sufentanil aerosol. However, the present study used a larger number of males than females so additional studies with greater statistical power to detect sex differences will be necessary to verify our current findings. These data add to the growing literature demonstrating the likely abuse potential of potent synthetic opioid aerosols. The present data also support the potential utility of nose-only opioid aerosol puff delivery in preclinical studies examining the abuse-related effects of drugs but considerable additional work is necessary to further refine rodent aerosol self-administration procedures before they can be considered an attractive alternative to intravenous self-administration.
Funding and/or Conflicts of interests/Competing interests
Funding provided by National Institute of Drug Abuse grant R21DA08150
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Awasaki Y, Nishida N, Sasaki S, Sato S, 1997. Dopamine D1 antagonist SCH23390 attenuates self-administration of both cocaine and fentanyl in rats. Environmental Toxicology and Pharmacology 3, 115–122. 10.1016/S1382-6689(97)00147-6 [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR, 1973. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav 20, 119–129. 10.1901/jeab.1973.20-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Krishnan-Sarin S, 2018. E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure. Curr Neuropharmacol 16, 438–459. 10.2174/1570159X15666171016164430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA, 2018. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob Res 20, 215–223. 10.1093/ntr/ntw280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacke Joan W., Bloor BC, Kripke BJ, Flacke WE, Warneck CM, Van Etten AP, Wong DH, Katz RL, 1985. Comparison of Morphine, Meperidine, Fentanyl, and Sufentanil in Balanced Anesthesia: A Double-Blind Study. Anesthesia & Analgesia 64, 897–910. [PubMed] [Google Scholar]
- Flacke JW, Bloor BC, Kripke BJ, Flacke WE, Warneck CM, Van Etten AP, Wong DH, Katz RL, 1985. Comparison of morphine, meperidine, fentanyl, and sufentanil in balanced anesthesia: a double-blind study. Anesth Analg 64, 897–910. [PubMed] [Google Scholar]
- Frie JA, Underhill J, Zhao B, de Guglielmo G, Tyndale RF, Khokhar JY, 2020. OpenVape: An Open-Source E-Cigarette Vapor Exposure Device for Rodents. eNeuro 7, ENEURO.0279–20.2020. 10.1523/ENEURO.0279-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Zimmermann ZJ, Baird TJ, 2018. The gold-standard in preclinical abuse liability testing: It’s all relative. Journal of Pharmacological and Toxicological Methods 94, 36–53. 10.1016/j.vascn.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Creehan KM, Taffe MA, 2021a. A vapor exposure method for delivering heroin alters nociception, body temperature and spontaneous activity in female and male rats. J Neurosci Methods 348, 108993. 10.1016/j.jneumeth.2020.108993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Nguyen JD, Creehan KM, Javadi-Paydar M, Grant Y, Taffe MA, 2021b. Effects of combined THC and heroin vapor inhalation in rats. Psychopharmacology (Berl). 10.1007/s00213-021-05904-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Nguyen JD, Creehan KM, Taffe MA, 2020. Female rats self-administer heroin by vapor inhalation. Pharmacol Biochem Behav 199, 173061. 10.1016/j.pbb.2020.173061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H, 2017. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl.) 234, 773–779. 10.1007/s00213-016-4512-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Muelken P, Smethells JR, Yershova K, Stepanov I, Olson TT, Kellar KJ, LeSage MG, 2018. Effects of nicotine-containing and “nicotine-free” e-cigarette refill liquids on intracranial self-stimulation in rats. Drug Alcohol Depend 185, 1–9. 10.1016/j.drugalcdep.2017.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvanko A, Kryscio R, Martin C, Kelly T, 2019. Stimulus effects of propylene glycol and vegetable glycerin in electronic cigarette liquids. Drug Alcohol Depend 194, 326–329. 10.1016/j.drugalcdep.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Sharpe LG, Jaffe JH, 1989. Rats self-administer sufentanil in aerosol form. Psychopharmacology (Berl) 99, 289–293. 10.1007/BF00445545 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Weinhold LL, Sharpe LG, 1990. Factors influencing self-administration of aerosol sufentanil in rats. NIDA Res Monogr 105, 382–383. [PubMed] [Google Scholar]
- Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, Taffe MA, 2019. Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug Alcohol Depend 198, 54–62. 10.1016/j.drugalcdep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA, 2018. Effects of Δ9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology (Berl) 235, 2541–2557. 10.1007/s00213-018-4946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Spindle TR, Gawron M, Sobczak A, Goniewicz ML, 2018. Nicotine emissions from electronic cigarettes: Individual and interactive effects of propylene glycol to vegetable glycerin composition and device power output. Food Chem Toxicol 115, 302–305. 10.1016/j.fct.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lee ES, Nguyen C, Zhu Y, 2020. Effects of propylene glycol, vegetable glycerin, and nicotine on emissions and dynamics of electronic cigarette aerosols. Aerosol Sci Technol 54, 1270–1281. 10.1080/02786826.2020.1771270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SG, Keller PS, Hammerslag LR, Bardo MT, 2021. Escalation and reinstatement of fentanyl self-administration in male and female rats. Psychopharmacology (Berl) 238, 2261–2273. 10.1007/s00213-021-05850-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kahn W, Cannon DG, Smith JE, 2006. Self-administration of heroin, cocaine and their combination under a discrete trial schedule of reinforcement in rats. Drug Alcohol Depend 82, 282–286. 10.1016/j.drugalcdep.2005.11.018 [DOI] [PubMed] [Google Scholar]
- McConnell SA, Brandner AJ, Blank BA, Kearns DN, Koob GF, Vendruscolo LF, Tunstall BJ, 2021. Demand for fentanyl becomes inelastic following extended access to fentanyl vapor self-administration. Neuropharmacology 182, 108355. 10.1016/j.neuropharm.2020.108355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliano C, Scott ER, Murdaugh LB, Gnatowski ER, Faunce CL, Anderson MS, Reyes MM, Gregus AM, Buczynski MW, 2020. Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems. J Neurosci Methods 330, 108458. 10.1016/j.jneumeth.2019.108458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Ortiz MM, Gantz SC, Tunstall BJ, Marchette RCN, Bonci A, Koob GF, Vendruscolo LF, 2020. Fentanyl vapor self-administration model in mice to study opioid addiction. Sci Adv 6, eabc0413. 10.1126/sciadv.abc0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder HA, Patterson JL, Halquist MS, Kosmider L, Turner JBM, Poklis JL, Poklis A, Peace MR, 2019. The Effect of Electronic Cigarette User Modifications and E-liquid Adulteration on the Particle Size Profile of an Aerosolized Product. Sci Rep 9, 10221. 10.1038/s41598-019-46387-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Cole M, Vandewater SA, Grant Y, Taffe MA, 2016a. Locomotor Stimulant and Rewarding Effects of Inhaling Methamphetamine, MDPV, and Mephedrone via Electronic Cigarette-Type Technology. Neuropsychopharmacology 41, 2759–2771. 10.1038/npp.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA, 2016b. Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109, 112–120. 10.1016/j.neuropharm.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Secci ME, Schindler CW, Bradberry CW, 2017. Choice between delayed food and immediate opioids in rats: treatment effects and individual differences. Psychopharmacology (Berl) 234, 3361–3373. 10.1007/s00213-017-4726-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PD, Nanding H, Strasser AA, Wise PM, 2018. Pilot Experiment: The Effect of Added Flavorants on the Taste and Pleasantness of Mixtures of Glycerol and Propylene Glycol. Chemosens Percept 11, 1–9. 10.1007/s12078-017-9231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Townsend EA, Orihuel J, Applebey SV, Claypool SM, Banks ML, Shaham Y, Negus SS, 2021. Lack of effect of different pain-related manipulations on opioid self-administration, reinstatement of opioid seeking, and opioid choice in rats. Psychopharmacology 238, 1885–1897. 10.1007/s00213-021-05816-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66, 1–11. 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Sharp JL, Ethridge SB, Ballard SL, Potter KM, Schmidt KT, Smith MA, 2021. The effects of chronic estradiol treatment on opioid self-administration in intact female rats. Drug Alcohol Depend 225, 108816. 10.1016/j.drugalcdep.2021.108816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Macenski MJ, Meisch RA, 1998. Reinforcing effects of a combination of ethanol and methadone relative to each drug alone. Pharmacol Biochem Behav 61, 367–374. 10.1016/s0091-3057(98)00100-2 [DOI] [PubMed] [Google Scholar]
- Shelton KL, Slavova-Hernandez G, 2009. Characterization of an inhaled toluene drug discrimination in mice: effect of exposure conditions and route of administration. Pharmacol. Biochem. Behav 92, 614–620. 10.1016/j.pbb.2009.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, Jacob P, Benowitz NL, 2016. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111, 535–544. 10.1111/add.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St.Helen G, Ross KC, Dempsey DA, Havel CM, Jacob P, Benowitz NL, 2016. Nicotine Delivery and Vaping Behavior During ad Libitum E-cigarette Access. Tob Regul Sci 2, 363–376. 10.18001/TRS.2.4.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustkova-Fiserova M, Puskina N, Havlickova T, Lapka M, Syslova K, Pohorala V, Charalambous C, 2020. Ghrelin receptor antagonism of fentanyl-induced conditioned place preference, intravenous self-administration, and dopamine release in the nucleus accumbens in rats. Addiction Biology 25, e12845. 10.1111/adb.12845 [DOI] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, Vandewater SA, Kerr TM, Cole M, 2021. Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Exp Clin Psychopharmacol 29, 1–13. 10.1037/pha0000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Thompson DF, 2016. Nebulized Fentanyl in Acute Pain: A Systematic Review. Ann Pharmacother 50, 882–891. 10.1177/1060028016659077 [DOI] [PubMed] [Google Scholar]
- van den Hoogen RH, Colpaert FC, 1987. Epidural and subcutaneous morphine, meperidine (pethidine), fentanyl and sufentanil in the rat: analgesia and other in vivo pharmacologic effects. Anesthesiology 66, 186–194. 10.1097/00000542-198702000-00013 [DOI] [PubMed] [Google Scholar]
- Vendruscolo JCM, Tunstall BJ, Carmack SA, Schmeichel BE, Lowery-Gionta EG, Cole M, George O, Vandewater SA, Taffe MA, Koob GF, Vendruscolo LF, 2018. Compulsive-Like Sufentanil Vapor Self-Administration in Rats. Neuropsychopharmacology 43, 801–809. 10.1038/npp.2017.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos N, Smith D, Kaiser L, Mahoney MC, Bradizza CM, Kozlowski LT, Benowitz NL, O’Connor RJ, Goniewicz ML, 2020. Effect of e-cigarette flavors on nicotine delivery and puffing topography: results from a randomized clinical trial of daily smokers. Psychopharmacology (Berl.) 237, 491–502. 10.1007/s00213-019-05386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang NS, Brown VL, Grabowski J, Meisch RA, 2001. Reinforcement by orally delivered methadone, cocaine, and methadone-cocaine combinations in rhesus monkeys: are the combinations better reinforcers? Psychopharmacology (Berl) 156, 63–72. 10.1007/s002130100731 [DOI] [PubMed] [Google Scholar]
- Weinhold LL, Sharpe LG, Jaffe JH, 1993. Housing conditions influence acquisition of sufentanil aerosol self-administration in rats. Pharmacol Biochem Behav 44, 141–144. 10.1016/0091-3057(93)90291-z [DOI] [PubMed] [Google Scholar]
- Yeadon M, Kitchen I, 1990. Multiple opioid receptors mediate the respiratory depressant effects of fentanyl-like drugs in the rat. Gen Pharmacol 21, 655–664. 10.1016/0306-3623(90)91013-h [DOI] [PubMed] [Google Scholar]


