Fig. 7.
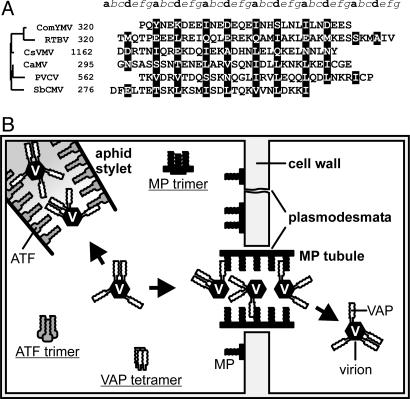
Prediction of Caulimoviridae MP coiled-coil domains and illustration of VAP-mediated CaMV functions. (A) MP C-terminal domains of representative members of the six genera of the Caulimoviridae family are aligned, the numbers representing amino acid position in each MP or polyprotein. The seven positions of the heptad repeat are labeled a-g above the alignment. Positions a and d, occupied by hydrophobic residues that are part of the oligomer core, are highlighted; the other positions are predominantly solvent-exposed polar residues. Sequences are aligned according to their phylogenetic relationships (shown on the left). Phylogeny was inferred from the intercellular transport movement domains located upstream of the coiled coils (corresponding to residues 127-157 in CaMV MP). Cassava vein mosaic virus, CsVMV; commelina yellow mottle virus, ComYMV; rice tungro bacilli-form virus, RTBV; soybean chlorotic mottle virus, SbCMV; petunia vein clearing virus, PVCV. (B) Schematic overview of VAP interactions mediating CaMV functions during the infection cycle. In the aphid stylet, VAP associated with the virion interacts with ATF to mediate aphid transmission. In the MP-modified plasmodesma, the VAP-virion complex interacts with MP, allowing movement to a neighboring cell. Also shown are MP and ATF trimers and VAP tetramers, which are known to occur in planta, although the function of these forms remains unknown. Interactions between the coiled coils of MP and VAP and ATF and VAP are shown only schematically and are not intended to depict the degree of coiled-coil multimerization.