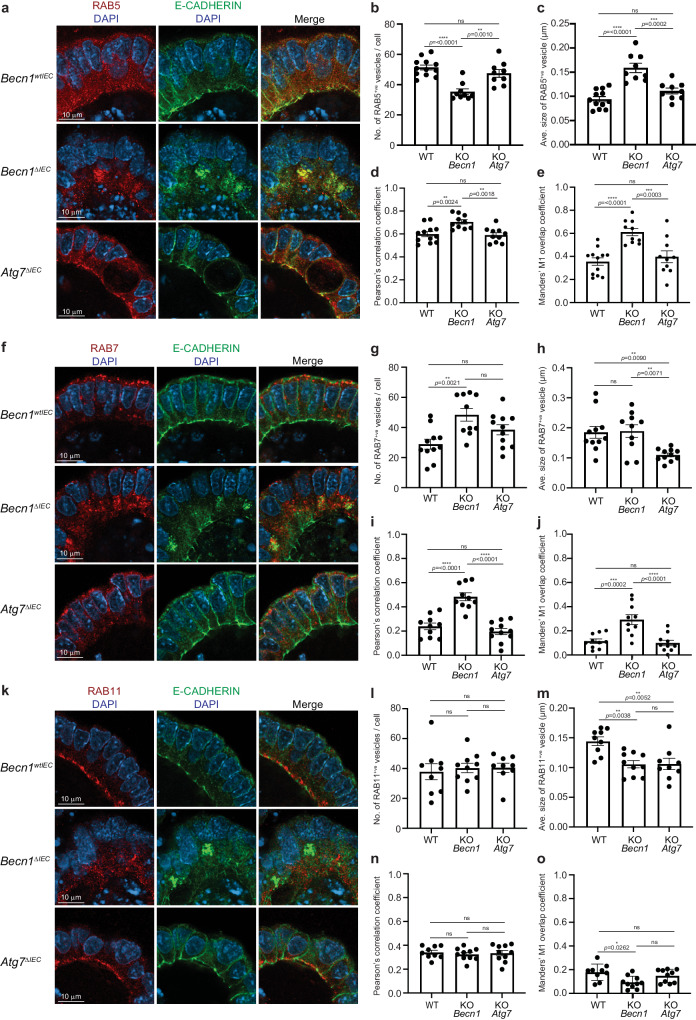
Fig. 5. BECLIN1 mediates the correct localization of E-CADHERIN through its regulation of endocytic trafficking.
a, f, k The absence of BECLIN1, but not ATG7, leads to loss of lateral, basal, and apical membrane staining of E-CADHERIN as detected by whole-mount immunofluorescent staining of wild-type, Becn1ΔIEC, and Atg7ΔIEC intestinal organoids. This is consistent with the increased intestinal permeability phenotype observed in Becn1ΔIEC mice (Fig. 1k). We also characterized the endocytic trafficking pathway by immunofluorescence staining of protein markers of the various components within the pathway, namely a–e RAB5+ve early endosomes, f–j RAB7+ve late endosomes, and k–o RAB11+ve recycling endosomes. In all Becn1ΔIEC-derived compartments, we observed mislocalisation of these vesicles with significant changes to the numbers and sizes of the vesicles in some compartments. Notably, the trapped E-CADHERIN within aberrantly large RAB5+ve early endosomes and its mislocalisation in BECLIN1-deficient intestinal epithelial cells can be attributed to defective endocytic trafficking. Data are representative of at least n = 3 different slices per organoid and of at least n = 3 biologically independent organoids from n = 3 independent experiments. Graphs indicate the mean ± S.E.M. Significance was determined by ordinary one-way ANOVA for endosomal numbers, size, and Pearson’s correlation coefficient and by two-way ANOVA for the Mander’s M1 overlap coefficient.