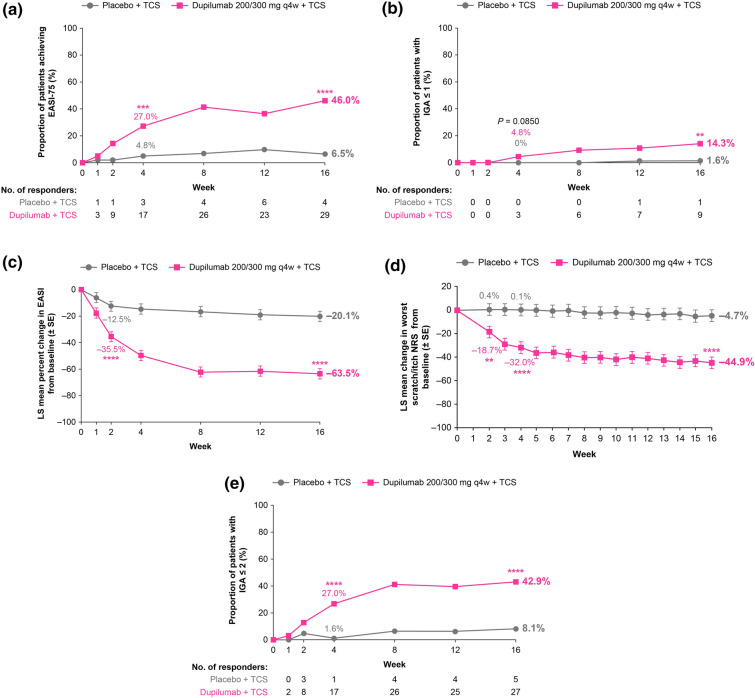
Fig. 1.
Primary and key secondary endpoints. a Proportion of patients with EASI-75 through week 16. b Proportion of patients with IGA ≤ 1 through to week 16. c LS mean percentage change in EASI from baseline through week 16. d LS mean percentage change in weekly mean of daily Worst Scratch and Itch NRS score from baseline through week 16. e Proportion of patients with IGA ≤ 2 through week 16. **P < 0.01; ***P < 0.001; ****P < 0.0001, all vs. corresponding placebo + TCS. EASI Eczema Area and Severity Index, EASI-75 75% decrease in EASI, IGA Investigator’s Global Assessment, LS least squares, NRS Numerical Rating Scale, q4w every 4 weeks, TCS topical corticosteroid