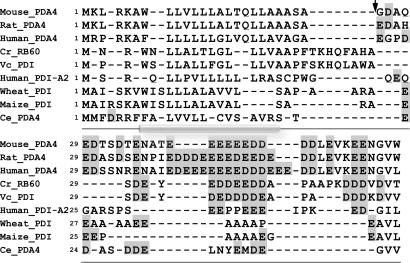
Fig. 4.
PDIs in a unique class contain acidic domains preceded by potential α-helix transmembrane domains at their N termini. Multiple alignment of the polypeptides was generated by using clustlw. Acidic amino acids are shaded. Alignment of the leader sequence of RB60 with that of other PDIs identified a small group of PDIs that contain a similar domain. The putative cleavage site for RB60 (marked by an arrow) was identified by using the computer program of Nielsen et al. (24). The secondary structure common to the leader of the aligned proteins (α-helix is denoted by cylindrical shading) was predicted by using tmap.