Abstract
G protein-mediated signaling is implicated in yeast and fungal cAMP pathways. By two-hybrid screens and pull-down experiments, we show that the fission yeast Gpa2 Gα binds an N-terminal domain of adenylate cyclase, comprising a moderately conserved sequence within a region otherwise poorly related to other fungal adenylate cyclases. Overexpressing this domain in yeast perturbs cAMP signaling, which is restored by Gpa2 coexpression. Mutations affecting this domain, over 1,100 residues from the catalytic domain, alter glucose-triggered cAMP signaling. This is evidence for direct activation of adenylate cyclase by a fungal G protein and suggests a distinct activation mechanism from that of mammals.
Keywords: cAMP, G protein, Schizosaccharomyces pombe
Heterotrimeric guanine-nucleotide binding proteins (G proteins), composed of Gα, Gβ, and Gγ subunits, couple to seven transmembrane helix receptors (G protein-coupled receptors or GPCRs) in the plasma membranes of eukaryotes, where they transduce extracellular signals into intracellular responses (1-3). Receptor-ligand binding events catalyze the exchange of GDP for GTP on the Gα subunit leading to dissociation from the βγ dimer. Both Gα subunits and βγ dimers are then able to regulate downstream effectors, including adenylate cyclase, cGMP phosphodiesterase (PDE), ion channels, mitogen-activated protein kinases (MAPK), phosphatidylinositol 3-kinase, and phospholipase C-β (2, 4). In mammals, G proteins, together with their cognate receptors and effectors, regulate complex signaling pathways that control processes including learning and memory, vision, taste and smell, tissue responses to hormone peptides, and muscle function (4).
Fungi share the same repertoire of signaling proteins as mammals; namely, G proteins, GPCRs, and effector enzymes, including adenylate cyclase and MAPK pathways, to sense nutritional availability and mating pheromones (5-7). These pathways are required for switching between yeast and filamentous growth forms (dimorphism) and for producing specialized structures for mating or penetrating host tissues (8, 9). In fungal systems, Gα subunits are required to properly regulate cAMP levels, presumably by directly regulating the activity of adenylate cyclase. Regulation of cAMP levels in medically and agriculturally important fungal pathogens is critical for virulence-factor induction and initiation of the infection process, yet the actual mechanism of this regulation is unknown (6-9).
Remarkably, the structural architecture of fungal adenylate cyclases is very different from that of mammalian adenylate cyclases, which are integral membrane proteins possessing a six-plus-six arrangement of transmembrane spanning helices and two cytoplasmic catalytic domains (10). Cocrystallization studies demonstrate that mammalian Gαs subunits directly contact both cytoplasmic catalytic domains of adenylate cyclase (11, 12). These results suggest an allosteric activation mechanism involving Gαs-mediated dimerization of the catalytic domains, which is essential for establishment of the ATP-binding site. Fungal adenylate cyclases share a conserved organization (Fig. 1A); but, in contrast to their mammalian counterparts, are peripheral membrane proteins with a single C-terminal catalytic domain (10). These enzymes possess a variable-length N-terminal region, followed by a domain, which, in the budding yeast Saccharomyces cerevisiae, interacts with the small GTP-binding protein Ras, although the Schizosaccharomyces pombe enzyme is not regulated by Ras (13, 14). The central core of fungal adenylate cyclases contains multiple leucine-rich repeat elements, followed by a protein phosphatase 2C-like domain and the catalytic domain (15). The very C terminus binds the cyclase-associated protein (CAP) in both budding and fission yeast (16, 17).
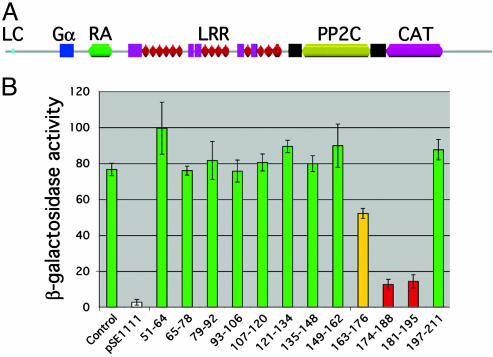
Fig. 1.
Mapping the Gpa2-binding domain of Schizosaccharomyces pombe adenylate cyclase. (A) Schematic of the pfam domain structure of Schizosaccharomyces pombe adenylate cyclase (pfam entry P14605 with location of Gpa2-binding domain added). LC, N-terminal low-complexity region (light-blue box); Gα, putative Gpa2-binding domain (blue square; residues 163-196); RA, Ras-activation domain (green; residues 292-372); LRR, leucine-rich repeats (magenta squares and red triangles; residues 430-961); PP2C, protein phosphatase 2C-like domain (yellow bar and flanking black squares; residues 1032-1268); CAT, catalytic domain (pink; residues 1323-1519). (B) Quantitative β-galactosidase assay results from internal deletion series analysis. Numbers indicate amino acids deleted from the original AC31-311 prey (Control). Green bars indicate deletion preys that retain the two-hybrid interaction with Gpa2R176H; red bars indicate deletion preys with significantly reduced two-hybrid interactions; the yellow bar indicates a deletion prey with a partially reduced interaction; and the white bar indicates a negative control construct (pSE1111) expressing a Snf4 prey (23) (units are mean specific activities of β-galactosidase ± SD of four independent cultures).
In Schizosaccharomyces pombe, extracellular glucose is sensed through a GPCR pathway triggering adenylate cyclase activation to produce a transient cAMP signal. This signal transduction cascade represses transcription of genes involved in sexual development and gluconeogenesis. Using reporter fusions to the fbp1+ gene, encoding the gluconeogenic enzyme fructose-1,6-bisphosphatase, we previously identified genes required for glucose detection and cAMP signaling. Five of these genes encode the Git2 adenylate cyclase (also known as Cyr1), the Git3 GPCR, the Gpa2 Gα, the Git5 Gβ, and the Git11 Gγ (14, 18-21). Because mutational activation of Gpa2 bypasses the need for the GPCR and Gβγ dimer, Gpa2 appears to be responsible for activation of adenylate cyclase (19, 21). We therefore sought to determine whether Gpa2 directly binds to and activates adenylate cyclase similar to mammalian cAMP pathways.
Materials and Methods
Yeast Strains, Media, and Growth Conditions. Schizosaccharomyces pombe strains used in this study are listed in Table 1. Standard Schizosaccharomyces pombe media recipes and strain construction methods are described in ref. 22. Saccharomyces cerevisiae strain YRG2 was purchased from Stratagene and maintained on rich medium (yeast extract-peptone-dextrose plus adenine) or synthetic defined dropout media (SD; Qbiogene).
Table 1. Strains used in this study.
| Strain | Genotype |
|---|---|
| FWP77 | h− leu1-32 ura4::fbp1-lacZ |
| CHP468 | h− fbp1::ura4+ ura4::fbp1-lacZ leu1-32 ade6-M210 his7-366 gpa2::ura4− |
| CHP578 | h+ fbp1::ura4+ ura4::fbp1-lacZ leu1-32 ade6-M210 his3-D1 |
| CHP733 | h+ ura4::fbp1-lacZ leu1-32 ade6-M216 cgs2-s1 |
| CHP748 | h+ fbp1::ura4+ ura4::fbp1-lacZ leu1-32 ade6-M210 his7-366 git2::LEU2+ cgs2-s1 |
| DIP16 | h− leu1-32 ura4::fbp1-lacZ git2::ura4+ |
| DIP17 | h− leu1-32 ura4::fbp1-lacZ git2+ |
| DIP22 | h− leu1-32 ura4::fbp1-lacZ git2Δ174-188 |
| DIP25 | h− leu1-32 ura4::fbp1-lacZ git2P180A |
| DIP30 | h− leu1-32 ura4::fbp1-lacZ git2P180A/P182A |
| DIP54 | h− leu1-32 ura4::fbp1-lacZ git2L177A/T178A |
| DIP62 | h− leu1-32 ade6-M210 ura4::fbp1-lacZ git2P180A cgs2-s1 |
| DIP63 | h− leu1-32 ade6-M210 ura4::fbp1-lacZ git2L177A/T178A cgs2-s1 |
| DIP66 | h− leu1-32 ade6-M210 ura4::fbp1-lacZ git2Δ174-188 cgs2-s1 |
Construction of a Random Adenylate Cyclase Fragment Prey Library. Plasmid pCHY26 (14) was digested with BsiWI, and the 5-kb DNA fragment containing the adenylate cyclase ORF was isolated. Random fragments of ≈1 kb were generated by partial MseI digestion and exonuclease BAL-31 treatment. After Klenow fill-in, BclI linkers were added by ligation, and the BclI-digested fragments were inserted into XhoI-linearized pACT2 (23) by using a partial fill-in strategy (24). This library was introduced into Escherichia coli, generating 30,000 primary transformants.
Yeast Two-Hybrid Bait Plasmid Construction and Library Screen. Bait plasmids were constructed that express either wild-type Gpa2+ or a GTPase-deficient “activated” Gpa2R176H fused to the Gal4 DNA-binding domain. Both bait plasmids were constructed by gap-repair cloning (25). PCR primers Gpa2-bait-for (5′-GT TCCAGAT TACGCTAGCT TGGGTGGTCATATGGCCATGGAGGCCATGACGATTTTTAATGGATTATCTGAAAGCG-3′) and Gpa2-bait-rev (5′-AAGAAATTCGCCCGGA AT TAGCT TGGCTGCAGGTCG ACGGATCCCTTAAAACATTCCCGCTTCTTTCAGACTGTG-3′) were used to amplify cDNA clones of these alleles, and the products were cotransformed into Saccharomyces cerevisiae YRG2 cells along with the SmaI-linearized bait vector pAS2 (23). Plasmids were recovered from transformants into E. coli XL1-Blue (26) and confirmed by DNA restriction analysis.
Saccharomyces cerevisiae YRG2 cells possessing either the Gpa2+ or Gpa2R176H bait plasmid were transformed with the git2+ prey library and plated onto SD-his-leu-trp plates. Colonies were screened by X-Gal filter lift to identify those expressing β-galactosidase activity. Candidate transformants were grown on medium containing tryptophan to allow loss of bait plasmids and were retested for β-galactosidase activity. Prey plasmids from transformants whose β-galactosidase activity was bait plasmid-dependent were rescued into E. coli (26) and subjected to DNA sequence analysis.
Prey Construct Truncation Analysis. Truncation derivatives of the prey plasmid pRH3 (AC33-311) were constructed by using BAL-31. First, an AscI linker (self-annealed, 5′-phosphorylated primer; 5′-TAAATAAATAAGGCGCGCCTTATTTATTTA-3′) containing stop codons in all six reading frames was inserted by ligation into the StuI site of plasmid pRH3 to create pDI64, which was used to clone BAL-31 truncated fragments of git2. For the production of truncated products, pRH3 was digested with SpeI, which cuts in codon 264, and incubated with BAL-31. The DNA ends were blunted by Klenow, and the AscI linker was added by ligation. This DNA was digested with NcoI and AscI and these fragments were inserted into NcoI/AscI-digested pDI64. Independent clones expressing truncated products were identified by agarose gel electrophoresis and assayed for interaction with the Gpa2R176H bait.
Site-directed mutagenesis, using the QuikChange Multi site-directed mutagenesis kit (Stratagene) with 5′-phosphorylated primers and template pRH3, was used to produce a series of consecutive 14 or 15 codon deletions as indicated in Fig. 1B or to make the following alanine substitutions: D167A, N169A/L170A/N171A, L177A/T178A, P180A, P180A/P182A, and S184A.
git2+ Allele Replacement. Unmarked mutations were introduced into the genomic git2+ locus by first disrupting git2+ with ura4+ and then knocking in the mutant alleles by selecting for the loss of the ura4+ marker. The git2+ disruption cassette was made by PCR amplification of ura4+ from plasmid pUC8-ura4 (27), using primers git2-ura4-for (5′-TAATTGTGCAATTTCTAAAGA AGCAGGCCT TCGA AGACT TAT TGATA AGGACAGAGAATCTTTCGACAAAGCTTAGCTACAAATCCCACTG-3′) and git2-ura4-rev (5′-TACCTTCCAAACTAGCTGGCCAATTGTTATTAAGAGAATCACGTTGAAACTCCACACTGTCACTGTCATCACGCAAACAAGGCATCGACT-3′). This PCR product was used to transform Schizosaccharomyces pombe strain FWP77 to Ura+ (28). X-Gal filter lifts identified Ura+ colonies lacking adenylate cyclase activity causing increased fbp1-lacZ expression (22). Candidate git2::ura4+ strains were confirmed by PCR analysis of genomic DNA. Strain DIP16 (git2::ura4+) was the host strain for subsequent allele replacement transformations.
PCR primers git2-knockin-for (5′-AGGACGATGACTCAGCGACGTCTG-3′) and git2-knockin-rev (5′-GGAAGTTCGATCATCACGGTATAC-3′) were used to amplify either git2+ or git2 mutant alleles containing mutations of interest from the two-hybrid analysis. These PCR products were used to transform strain DIP16 (git2::ura4+) to 5-fluoroorotic acid resistance, replacing the ura4+ marker with the PCR product by homologous recombination. The recombination event was verified by PCR, and the diagnostic PCR product was sequenced to confirm that only the desired mutation was present in the endogenous git2 locus.
Cloning, Overexpression, and AC1-200V5H6/HA-Gpa2 Copurification. Plasmid pDI66, expressing the first 200 residues of adenylate cyclase from the nmt1 promoter, was constructed by PCR amplification of plasmid pCHY26 (14) DNA with primers 1-200-for (5′-ATGGATCAAAGCAAGCGAAT-3′) and 1-200-rev (5′-ATTGTTATTAAGAGAATCACGTTG-3′), and TOPO cloning into the SpECTRA Schizosaccharomyces pombe Expression System vector pNMT1 TOPO TA (Invitrogen). The insert was verified by DNA sequence analysis. Plasmid pDI115, expressing influenza hemagglutinin epitope (HA)-tagged Gpa2 from the nmt41 promoter was constructed by amplifying gpa2+ cDNA on plasmid pDI2 with primers HA-GPA2-for (5′-ATGTACCCATACGACGTCCCAGACTACGCGACGATTTTTAATGGATTATCTGAAAGCG-3′) and gpa2-rev-STOP (5′-TTAAAACATTCCCGCTTCTTTCAGACTGTG-3′). The PCR product was cloned into pNMT41 TOPO TA (Invitrogen). Plasmid pDI136 is a his3+-marked derivative of pDI115 created by gap-repair marker swap (29).
For coexpression of AC1-200V5H6 and HA-Gpa2, Schizosaccharomyces pombe strain CHP578 was cotransformed with pDI66 and pDI136, and cells were plated onto medium containing 10 μg/ml thiamine to repress transcription from nmt promoters (30). Transformants were screened for expression of both recombinant proteins by Western analysis, using the α-V5-horseradish peroxidase-conjugated antibody (α-V5-HRP at 1:5,000; Invitrogen) and the α-HA antibody (1:2,000; Santa Cruz Biotechnology). Goat α-mouse IgG-HRP (1:10,000) was used to detect the α-HA antibody (Kirkegaard & Perry Laboratories).
For preparation of whole-cell extracts from cells expressing both AC1-200V5H6 and HA-Gpa2, or HA-Gpa2 alone (negative control), 10-ml cultures were grown for 16 h in medium lacking thiamine to induce nmt-driven expression. These cultures were inoculated into 150 ml of the same medium and grown overnight to a final cell density of ≈3 × 107 cells per ml. Cultures were harvested by centrifugation, washed with ice-cold extraction buffer [50 mM NaPi, pH 8/0.3 M NaCl/5% (vol/vol) glycerol/0.33 mM PMSF], and resuspended in 12 ml of extraction buffer. Total cell protein was extracted by 10 passages through a microfluidizer (Microfluidics). Cell lysates were centrifuged at 3,000 × g for 10 min at 4°C, and the supernatant was collected. The nonhydrolyzable GTP-analog GppNHp was added (final concentration 100 μM), as was a single EDTA-free protease inhibitor mixture tablet (Roche Diagnostics). The extract was adjusted to 1.5 mg/ml, and 10 ml of this solution added to a 0.25-ml bed volume equivalent of buffer-equilibrated TALON resin (BD Biosciences Clontech). Binding reactions were incubated at room temperature with mixing for 30 min. The flow-through (FT) was collected, and the column was washed with 10 ml of ice-cold extraction buffer followed by a wash with 10 ml of extraction buffer containing 0.5 M NaCl. Bound proteins were eluted by using extraction buffer containing 150 mM imidazole. Protein fractions were separated on 15% SDS/PAGE gels and analyzed for the presence of the HA-Gpa2 and/or AC1-200V5H6 proteins by using α-HA and α-V5-HRP antibodies as described above.
β-Galactosidase Assays. Schizosaccharomyces pombe and Saccharomyces cerevisiae cell extracts were assayed for β-galactosidase activity as described in ref. 18.
cAMP Assays. cAMP assays were conducted as described in ref. 31 with the following modifications. Cells were first grown in filter-sterilized, Edinburgh minimal medium (EMM; Qbiogene) containing 3% glucose to a density of 5 × 106 to 1 × 107, then subcultured to an initial concentration of 1.5 × 106 cells per ml for 24 h in prewarmed, filter-sterilized EMM media containing 0.1% glucose and 3% glycerol. Cultures typically reached a final density of 107 cells per ml.
DNA and Protein Sequence Analysis. DNA sequencing was performed by using the CEQ Quick Start dye terminator cycle sequencing kit (Beckman Coulter). The pfam image (32) in Fig. 1 A was obtained from the Sanger Institute genedb. Protein sequence alignments were performed by using clustalw (33) and presented by using boxshade 3.21 (www.ch.embnet.org). The organism names and GenBank protein database accession numbers used in Fig. 2 are as follows: Schizosaccharomyces pombe, CAA19571; Saccharomyces cerevisiae, CAA89295; Blumeria graminis, CAC19663; Botryotinia fuckeliana, CAB77164; Magnaporthe grisea, AAC34139; Podospora anserina, JC4747; Gibberella zeae PH-1, EAA68095; Metarhizium anisopliae, CAB64345; Colletotrichum lagenarium, BAD04045; Neurospora crassa, Q01631; Aspergillus fumigatus, CAC81748; Paracoccidioides brasiliensis, AAS01025; Candida albicans, AAG18428; Cryptococcus neoformans, AAG60619.
Fig. 2.
Alignment of the putative Gpa2-binding domain from Git2 with sequences of other fungal adenylate cyclases. Plus and minus signs indicate the positions of nine conserved residues in Git2 subjected to alanine substitution and the resulting effect on the ability to interact (+) or not (-) with the Gpa2R176H bait in the two-hybrid assay. The residue numbers follow each entry used in the alignment along with the size of the adenylate cyclase protein in parentheses.
Results
Two-Hybrid Identification of a Gpa2-Binding Domain in Adenylate Cyclase. A yeast two-hybrid screen was used to identify a candidate Gpa2-binding domain within adenylate cyclase. Bait plasmids expressing Gal4-binding domain fusions to either wild-type Gpa2+ or mutationally activated Gpa2R176H, predicted to be in the effector-binding conformation, were cotransformed with a prey library expressing random fragments of adenylate cyclase fused to the Gal4 activation domain. Approximately 1% of transformants displayed enhanced β-galactosidase expression from the lacZ reporter, indicative of a protein-protein interaction. Sixteen candidate prey plasmids were recovered and retested against both bait plasmids. Sequence analysis revealed that 13 of these prey plasmids contained in-frame translational fusions of the git2 ORF with the Gal4 activation domain ORF. Among these, 10 represented unique clones and all contained the N-terminal coding region of git2+. The 5′ git2+ codons in this collection ranged from 33 to 65, and the 3′ codons ranged from 258 to 311. Consistent with a biologically significant interaction, the activated Gpa2R176H bait displays a higher affinity than the wild-type Gpa2+ bait for any given prey as judged by β-galactosidase expression from the lacZ reporter. For example, the average β-galactosidase value for the Gpa2R176H bait interacting with five independent preys is 74 ± 16 relative to 43 ± 12 units for the wild-type Gpa2 bait (data not shown). Thus, we appear to have identified a direct interaction between Schizosaccharomyces pombe Gpa2 and adenylate cyclase.
Subsequent analyses were carried out to define the Gpa2-binding domain within adenylate cyclase. Starting from the largest adenylate cyclase prey containing codons 33-311 (AC33-311), a C-terminal deletion series was constructed. Twenty-four truncated derivatives were isolated and screened for an interaction with the Gpa2R176H bait. Preys containing residues 33-196 or more of adenylate cyclase retained their interaction with Gpa2, whereas preys containing residues 33-180 or fewer did not. This result was further verified by constructing a series of site-directed internal deletion derivatives that each progressively removed 14-15 codon segments from the AC33-311 prey plasmid beginning at codon 51. Consistent with the truncation analysis, deletions that removed either residues 174-188 or 181-197 significantly reduce Gpa2 binding as judged by a quantitative β-galactosidase assay (Fig. 1B). Western blot analyses using α-HA antibodies (the pACT2 vector adds an HA epitope tag to the cloned product) revealed that these various constructs express prey proteins at similar levels (data not shown), indicating that differences in the strength of these interactions do not simply reflect differences in protein stability.
The N terminus of Schizosaccharomyces pombe adenylate cyclase is largely unrelated to those of other fungal adenylate cyclases; however, residues 167-184 of Git2 appear to contain a conserved element within these proteins (Fig. 2). Alanine-substitution mutagenesis of the AC33-311 prey at nine of the most highly conserved positions within this region revealed that substitution of P180, P180 and P182, or L177 and T178 significantly reduced the interaction with the Gpa2R176H bait in the two-hybrid assay. Specifically, the P180A mutation resulted in 10 ± 1 β-galactosidase units, the P180A/P182A mutation resulted in 20 ± 3 units, and the L177/T178 mutation resulted in 12 ± 2 units compared with the wild-type prey value of 77 ± 4 units. Substitution of conserved residues to either side of these residues had little or no effect in this assay (residues indicated by plus signs in Fig. 2). Because substitution of these four amino acids significantly reduces the Gpa2-adenylate cyclase interaction, this sequence may represent a fungal adenylate cyclase Gα-binding domain.
Functional and Physical Demonstration of Adenylate Cyclase-Gpa2 Interaction in S. pombe Cells. To examine the biological role of this putative Gpa2-binding domain, we overexpressed the first 200 residues of adenylate cyclase (AC1-200V5H6, also carrying a V5 epitope and hexahistidine tag) in a Schizosaccharomyces pombe strain possessing two reporter genes that serve as readouts of glucose detection and cAMP signaling. The reporters contain the glucose-repressible fbp1+ promoter driving expression of the Schizosaccharomyces pombe ura4+ gene, required for uracil biosynthesis, and the E. coli lacZ gene, encoding β-galactosidase. When grown in the presence of glucose, cells with a functioning cAMP pathway repress expression of these reporters to inhibit growth on medium lacking uracil, while allowing growth in the presence of the pyrimidine analog 5-fluoroorotic acid (22). These cells also express low levels of β-galactosidase activity. However, cells expressing AC1-200V5H6 form colonies on medium lacking uracil, fail to grow in 5-fluoroorotic acid-containing medium, and express high β-galactosidase activity (Fig. 3A). These phenotypes are indicative of a defect in cAMP signaling because a large mutant selection for such strains has only led to the identification of genes encoding components of the cAMP signaling pathway (14, 18, 20-22, 31, 34). The defect in cAMP signaling seen here appears to be due to titration of Gpa2 by AC1-200V5H6, because co-overexpression of Gpa2 restores glucose repression of these reporter genes.
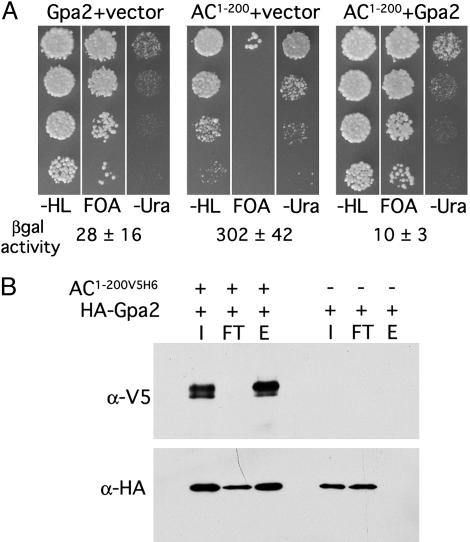
Fig. 3.
Gpa2 functionally interacts with the Git2 N terminus. (A) Phenotypic analysis of fbp1-ura4 reporter gene expression in a wild-type Schizosaccharomyces pombe strain transformed with plasmids expressing AC1-200V5H6, Gpa2, or both, as indicated. Serial dilution series of logarithmic phase cells from liquid cultures containing 8% glucose (wt/vol) were spotted onto 3% glucose medium lacking leucine and histidine (-HL; to maintain plasmid selection) and incubated at 30°C for 16 h before replica-plating to media containing 5-fluoroorotic acid (FOA) or lacking uracil (-Ura). Plates were photographed after a 48-h incubation. Numbers below the images indicate the mean specific activity of β-galactosidase ± SD of three to four independent cultures grown in high glucose (8% wt/vol) medium. (B) Schizosaccharomyces pombe whole-cell extracts were prepared from a wild-type strain expressing AC1-200V5H6 and HA-Gpa2 or HA-Gpa2 alone, as indicated, and incubated with immobilized Co2+-Sepharose beads. Input (I), flow-through (FT; unbound), and elution (E) fractions were examined by immunoblot to detect AC1-200V5H6 (α-V5) and HA-Gpa2 (α-HA).
To directly test for a physical interaction between Gpa2 and AC1-200V5H6, a pull-down/copurification experiment was carried out in Schizosaccharomyces pombe cells expressing a functional, HA epitope-tagged Gpa2 along with AC1-200V5H6 (Fig. 3B). The hexahistidine-tagged AC1-200V5H6 polypeptide from cell lysates was bound to a Co2+ resin (see Materials and Methods), and as shown in Fig. 3B, HA-Gpa2 was copurified. In cells not expressing AC1-200V5H6, HA-Gpa2 did not bind to the resin. Consistent with our two-hybrid results, HA-Gpa2 copurifies with AC1-200V5H6 when Schizosaccharomyces pombe cell lysates are preincubated with either the GTP-analog GppNHp (Fig. 3B) or GDP-βS (data not shown). Thus, Gpa2 binds adenylate cyclase in both its GDP and GTP-bound form.
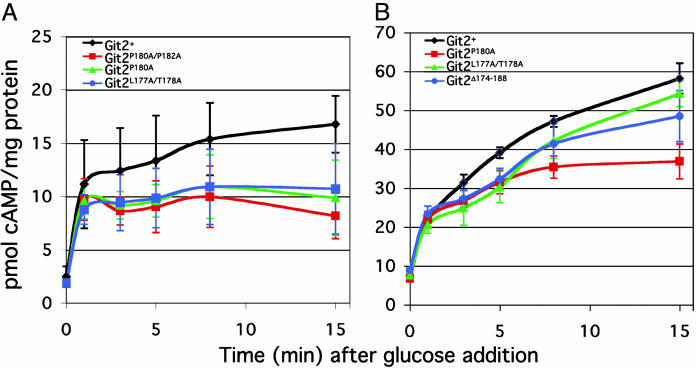
Chromosomal Mutations Targeting the Gpa2-Binding Domain of the Adenylate Cyclase Gene Confer cAMP-Signaling Defects. To examine the function of the Gpa2-binding domain within the context of the full-length adenylate cyclase enzyme, an allele replacement strategy was used to target specific mutations to the endogenous git2+ locus in Schizosaccharomyces pombe. Initial characterization of these strains indicated that the fbp1+-reporter constructs remained glucose-repressible (data not shown). Thus, no gross change in cAMP signaling occurred. To test for more subtle effects on the cAMP response to glucose, we directly measured the glucose-triggered cAMP signal in these strains (Fig. 4A). The cAMP levels in these strains are indistinguishable from those of a control strain for both basal levels and the response level at the 1-min time point after glucose addition. However, the mutant strains fail to sustain or fully mount a cAMP response. Both git2P180A and git2P180A/P182A strains consistently fail to accumulate the same level of cAMP observed in wild-type cells, although the defect is modest (Fig. 4A). Because the intracellular cAMP level is partially controlled through feedback regulation due to activation of cAMP PDE (L. Wang, F.D.I., and C.S.H., unpublished observations), a defect in cAMP synthesis in these strains may be partially masked by a compensatory reduction in PDE activation. We therefore crossed some git2 mutant alleles (git2P180A, git2L177A/T178A, and git2Δ174-188) into a PDE mutant background (cgs2-s1) and reexamined the cAMP response to glucose (Fig. 4B). Consistent with previous measurements, the initial response is similar to that of the control strain but altered in subsequent time points. Remarkably, the git2P180A mutation results in a 20 pmol/mg protein reduction, relative to wild-type cells, in the cAMP signal at the 15-min time point (Fig. 4B). Therefore, these substitutions affect the ability of the cell to reach or maintain full adenylate cyclase activation after the initial response to glucose. Because deletion of the gpa2 Gα gene causes a reduction in basal cAMP levels and glucose-stimulated cAMP levels (35), these mutations may not fully disrupt the Gpa2 interaction with adenylate cyclase in the context of the full-length enzyme. Alternatively, Gpa2 may have an additional role in adenylate cyclase activation that is unaffected by mutations in this domain.
Fig. 4.
Substitution mutations in the adenylate cyclase Gpa2-binding domain alter glucose-triggered cAMP signaling. (A) Glucose-stimulated cAMP response profiles in glucose-starved Schizosaccharomyces pombe cultures. The indicated cultures were grown for 24 h to a density of ≈107 cells per ml in the presence of 3% glycerol/0.1% glucose before the addition of glucose (to 100 mM final concentration). (B) Measurement of glucose-stimulated cAMP accumulation in Schizosaccharomyces pombe cells containing the cgs2-s1 PDE allele together with site-directed mutations in git2.
Discussion
This study provides evidence of a fungal Gα directly binding and activating adenylate cyclase, consistent with genetic studies in several organisms that implicate Gα-mediated activation of adenylate cyclases. Because Gα subunits activate mammalian adenylate cyclases by binding the catalytic domains, it was unexpected that the Gpa2-binding domain in the Schizosaccharomyces pombe enzyme would be ≈1,100 residues from the catalytic domain. However, most residues in the mammalian enzymes are in the form of transmembrane domains, and the catalytic domains may represent the only regions exposed to the cytoplasm for Gα to bind. Fungal adenylate cyclases are only peripherally associated with the plasma membrane, allowing Gα subunits to bind other regions of these enzymes, thus conserving the functional relationship between these proteins while allowing divergence of the activation mechanism.
Our studies suggest that we have identified a direct physical interaction between Gpa2 and adenylate cyclase. Although it had been possible that Gpa2 could have bound an intermediate protein that made contact with adenylate cyclase, it is unlikely that Saccharomyces cerevisiae would express a similar protein that would be localized to the nucleus where it could facilitate the two-hybrid interaction. In addition, it would be unlikely that co-overexpression of Gpa2 would restore glucose repression of the fbp1-driven reporters (Fig. 3A) in cells overexpressing AC1-200V5H6 if Gpa2 were not directly binding the AC1-200V5H6 polypeptide.
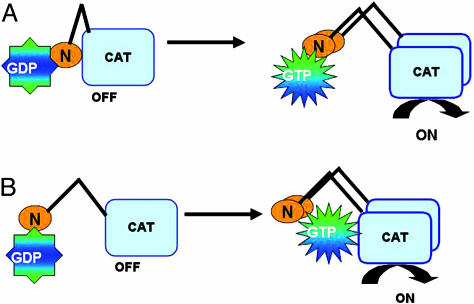
It remains to be determined how Gpa2 binding to adenylate cyclase results in effector activation. The adenylate cyclase N terminus may contain a region that inhibits catalytic activity with the binding of Gpa2-GTP to this domain, alleviating this effect (Fig. 5A). This model is supported by the observation of constitutive activation of Saccharomyces cerevisiae adenylate cyclase in strains expressing the C-terminal half of the enzyme and the finding that this activated form of the enzyme is insensitive to guanine nucleotides (36). Because this N-terminal Gpa2-binding domain strongly autoactivates two-hybrid reporter expression (F.D.I., unpublished observations), we have thus far been unable to test this model by two-hybrid screen to determine whether this domain interacts with other domains of adenylate cyclase. Alternatively, the Gpa2-binding domain may represent an entry point for contact between Gpa2-GDP and adenylate cyclase before signaling. In response to glucose detection and GTP-charging of Gpa2, the activated Gpa2 would then be positioned to contact the adenylate cyclase catalytic domain (Fig. 5B). This model would allow for an activation mechanism similar to that seen in mammals in which Gα subunits facilitate adenylate cyclase catalytic domain dimerization and stabilization of the active conformation (11). We should note that in Saccharomyces cerevisiae, both Ras proteins and a Gα subunit encoded by GPA2+ have been implicated in adenylate cyclase activation, although only the Ras proteins have been shown to directly bind adenylate cyclase (37-39). As shown in Fig. 1A, the Ras-binding domain within Saccharomyces cerevisiae adenylate cyclase is also an N-terminal domain; however, it is distinct from the Gpa2-binding domain identified in this study. As with Schizosaccharomyces pombe Gpa2, it remains to be determined how Saccharomyces cerevisiae Ras binding to adenylate cyclase leads to increased cAMP signaling. Given the structural conservation of fungal adenylate cyclases and Gα subunits, the elucidation of the mechanism by which Schizosaccharomyces pombe Gpa2 activates adenylate cyclase should enhance our understanding of cAMP signaling in fungal pathogens, many of which use this signaling pathway to regulate virulence factors.
Fig. 5.
Two models for Gpa2 regulation of Schizosaccharomyces pombe adenylate cyclase. (A) Gpa2, in its GDP- or GTP-bound form, binds the N-terminal domain of adenylate cyclase. GTP loading onto Gpa2 triggers a change in adenylate cyclase to overcome an inhibitory effect of the N terminus on the catalytic domain at the C terminus. (B) GDP-Gpa2 binding to adenylate cyclase facilitates binding of the activated GTP-Gpa2 to both the N-terminal domain and to a site within the catalytic domain that stimulates adenylate cyclase activity.
Acknowledgments
We thank A. M. Kays and members of C.S.H.'s laboratory for helpful discussions and critical review of the manuscript. This work was supported by National Institutes of Health (NIH) Grant GM46226 (to C.S.H.) and NIH National Research Service Award GM068399 (to F.D.I.).
Author contributions: F.D.I. and C.S.H. designed research; F.D.I. performed research; F.D.I. and C.S.H. analyzed data; and F.D.I. and C.S.H. wrote the paper.
Abbreviations: GPCR, G protein-coupled receptor; HA, influenza hemagglutinin epitope; PDE, phosphodiesterase.
References
- 1.Hepler, J. R. & Gilman, A. G. (1992) Trends Biochem. Sci. 17, 383-387. [DOI] [PubMed] [Google Scholar]
- 2.Simon, M. I., Strathmann, M. P. & Gautam, N. (1991) Science 252, 802-808. [DOI] [PubMed] [Google Scholar]
- 3.Gilman, A. G. (1987) Annu. Rev. Biochem. 56, 615-649. [DOI] [PubMed] [Google Scholar]
- 4.Neves, S. R., Ram, P. T. & Iyengar, R. (2002) Science 296, 1636-1639. [DOI] [PubMed] [Google Scholar]
- 5.Kays, A. M. & Borkovich, K. A. (2004) in The Mycota III, eds. Brambl, R. & Marzluf, G. A. (Springer, Berlin), pp. 175-207.
- 6.Lengeler, K. B., Davidson, R. C., D'Souza, C., Harashima, T., Shen, W. C., Wang, P., Pan, X., Waugh, M. & Heitman, J. (2000) Microbiol. Mol. Biol. Rev. 64, 746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Souza, C. A. & Heitman, J. (2001) FEMS Microbiol. Rev. 25, 349-364. [DOI] [PubMed] [Google Scholar]
- 8.Borges-Walmsley, M. I. & Walmsley, A. R. (2000) Trends Microbiol. 8, 133-141. [DOI] [PubMed] [Google Scholar]
- 9.Bolker, M. (1998) Fungal Genet. Biol. 25, 143-156. [DOI] [PubMed] [Google Scholar]
- 10.Tang, W. J. & Gilman, A. G. (1992) Cell 70, 869-872. [DOI] [PubMed] [Google Scholar]
- 11.Tesmer, J. J., Sunahara, R. K., Gilman, A. G. & Sprang, S. R. (1997) Science 278, 1907-1916. [DOI] [PubMed] [Google Scholar]
- 12.Zhang, G., Liu, Y., Ruoho, A. E. & Hurley, J. H. (1997) Nature 386, 247-253. [DOI] [PubMed] [Google Scholar]
- 13.Fukui, Y., Kozasa, T., Kaziro, Y., Takeda, T. & Yamamoto, M. (1986) Cell 44, 329-336. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman, C. S. & Winston, F. (1991) Genes Dev. 5, 561-571. [DOI] [PubMed] [Google Scholar]
- 15.Baker, D. A. & Kelly, J. M. (2004) Mol. Microbiol. 52, 1229-1242. [DOI] [PubMed] [Google Scholar]
- 16.Kawamukai, M., Gerst, J., Field, J., Riggs, M., Rodgers, L., Wigler, M. & Young, D. (1992) Mol. Biol. Cell 3, 167-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, J., Suzuki, N., Nishida, Y. & Kataoka, T. (1993) Mol. Cell. Biol. 13, 4087-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nocero, M., Isshiki, T., Yamamoto, M. & Hoffman, C. S. (1994) Genetics 138, 39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landry, S. & Hoffman, C. S. (2001) Genetics 157, 1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landry, S., Pettit, M. T., Apolinario, E. & Hoffman, C. S. (2000) Genetics 154, 1463-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welton, R. M. & Hoffman, C. S. (2000) Genetics 156, 513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman, C. S. & Winston, F. (1990) Genetics 124, 807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. (1993) Cell 75, 805-816. [DOI] [PubMed] [Google Scholar]
- 24.Barbet, N., Muriel, W. J. & Carr, A. M. (1992) Gene 114, 59-66. [DOI] [PubMed] [Google Scholar]
- 25.Muhlrad, D., Hunter, R. & Parker, R. (1992) Yeast 8, 79-82. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman, C. S. & Winston, F. (1987) Gene 57, 267-272. [DOI] [PubMed] [Google Scholar]
- 27.Bach, M. L. (1987) Curr. Genet. 12, 527-534. [DOI] [PubMed] [Google Scholar]
- 28.Bahler, J., Wu, J. Q., Longtine, M. S., Shah, N. G., McKenzie, A., III, Steever, A. B., Wach, A., Philippsen, P. & Pringle, J. R. (1998) Yeast 14, 943-951. [DOI] [PubMed] [Google Scholar]
- 29.Kelly, D. A. & Hoffman, C. S. (2002) BioTechniques 33, 978, 980, 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsburg, S. L. (1993) Nucleic Acids Res. 21, 2955-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrne, S. M. & Hoffman, C. S. (1993) J. Cell Sci. 105, 1095-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonnhammer, E. L., Eddy, S. R., Birney, E., Bateman, A. & Durbin, R. (1998) Nucleic Acids Res. 26, 320-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994) Nucleic Acids Res. 22, 4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schadick, K., Fourcade, H. M., Boumenot, P., Seitz, J. J., Morrell, J. L., Chang, L., Gould, K. L., Partridge, J. F., Allshire, R. C., et al. (2002) Eukaryot. Cell 1, 558-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isshiki, T., Mochizuki, N., Maeda, T. & Yamamoto, M. (1992) Genes Dev. 6, 2455-2462. [DOI] [PubMed] [Google Scholar]
- 36.Heideman, W., Casperson, G. F. & Bourne, H. R. (1990) J. Cell. Biochem. 42, 229-242. [DOI] [PubMed] [Google Scholar]
- 37.Harashima, T. & Heitman, J. (2004) in Nutrient Induced Responses in Eukaryotic Cells, eds. Winderickx, J. & Taylor, P. M. (Springer, Berlin), Vol. 7, pp. 131-69. [Google Scholar]
- 38.Thevelein, J. M. & de Winde, J. H. (1999) Mol. Microbiol. 33, 904-918. [DOI] [PubMed] [Google Scholar]
- 39.Versele, M., Lemaire, K. & Thevelein, J. M. (2001) EMBO Rep. 2, 574-579. [DOI] [PMC free article] [PubMed] [Google Scholar]