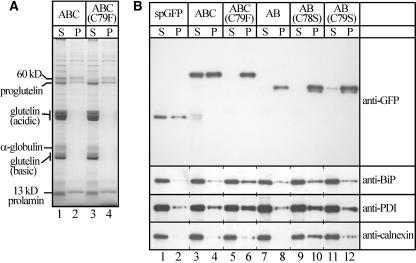
Figure 5.
The Roles of ER Chaperons in Sorting Fusion Proteins.
(A) Proteins were extracted from mature seeds with SDS buffer, followed by centrifugation (see procedures in Methods). Proteins in the supernatants (S) and pellets (P) were separated on a 12% SDS-PAGE gel and stained with Coomassie Brilliant Blue. Lanes 1 and 2, spG-GFP-ABC (Figure 1A); lanes 3 and 4, spG-GFP-ABC(C79F) (Figure 1G). Note that almost all α-globulin and processed glutelins (acidic and basic subunits) were present in the supernatant, whereas significant amounts of proglutelins were present in the pellet fraction.
(B) Distinct extractabilities of fusion proteins and protein chaperons with the SDS buffer. The proteins in the supernatants (S) and pellets (P) were separated on a 12% SDS-PAGE gel, followed by protein gel blot analyses with antibodies against GFP, BiP, PDI, and calnexin. Lanes 1 and 2, spGFP (Figure 8A); lanes 3 and 4, spG-GFP-ABC; lanes 5 and 6, spG-GFP-ABC(C79F); lanes 7 and 8, spG-GFP-AB (Figure 4A); lanes 9 and 10, spG-GFP-AB(C78S) (Figure 4B); lanes 11 and 12, spG-GFP-AB(C79S) (Figure 4C).