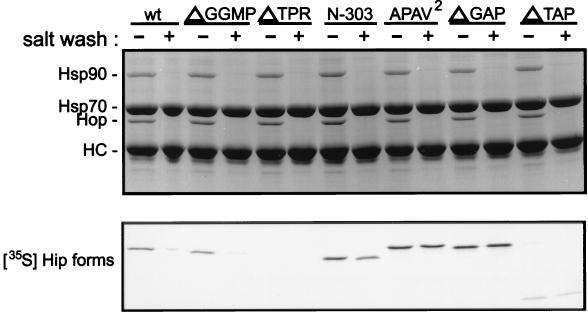
FIG. 3.
Coprecipitation of Hip forms with Hsp70. Hsp70 complexes were immunoprecipitated from RL supplemented with the radiolabeled Hip forms indicated above the lanes (wt, wtHip). An equimolar amount of each radiolabeled Hip form was added to separate mixtures for immunoprecipitation reactions. Immunoprecipitates were washed in buffer containing either no additional salt (−) or 0.5 M NaCl (+). Proteins in resin complexes were separated by SDS-PAGE and visualized by Coomassie blue staining (upper panel). Hsp70 and the coprecipitating proteins Hop and Hsp90 are indicated on the left. Hip is not evident in the stained gel due to its comigration with anti-Hsp70 BB70 heavy chain (HC). Coprecipitating radiolabeled Hip forms were detected by autoradiography of the dried gel (lower panel).