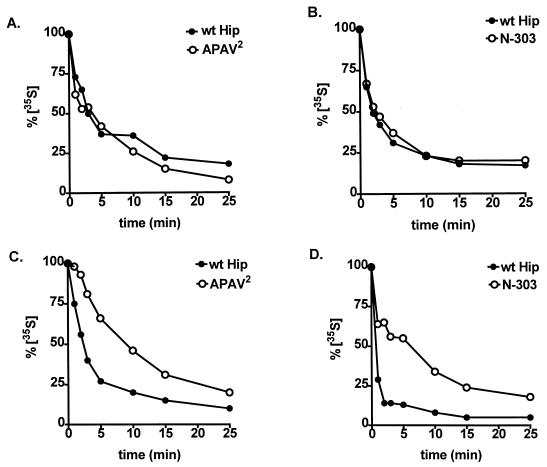
FIG. 8.
Effects of mutant Hip forms on the dynamics of Hsp70 interactions. The rates of exchange of radiolabeled Hsp70 on PR complexes (A and B) and on Hip complexes (C and D) were compared in the presence of wtHip (A to D), APAV2 (A and C), or N-303 (B and D). As detailed in Materials and Methods, PR or Hip complexes were assembled in RL containing radiolabeled Hsp70 and then transferred to fresh RL lacking radioactivity. At the times indicated, aliquots were removed from the secondary assemblies. Each sample was separated by SDS-PAGE, Coomassie blue stained to monitor total protein levels, and autoradiographed to measure the binding of radioactive Hsp70. Bands were quantitated by densitometry, and radioactive bands were normalized to the amount of Coomassie blue-stained Hsp70 in each sample. The resulting values are plotted as a percentage of radiolabeled Hsp70 present at initiation of the secondary assembly reactions (0 min).