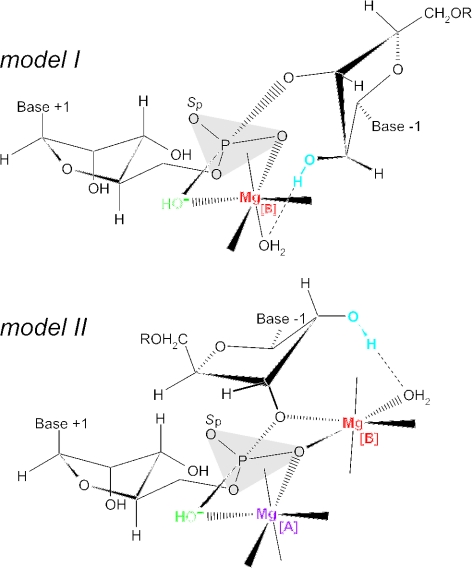
Figure 5.
Transition state models for phosphodiester hydrolysis by E.coli M1 RNA. The single 2′-OH at nt −1 (in blue) was replaced with a 2′-deoxy, 2′-amino or 2′-fluoro group in the modified substrates analyzed in Figure 6. Putative, catalytically important Mg2+ ions are shown in magenta (Mg [A]) or red (Mg [B]); metal ion site [B] was in the focus of the present study. The first transition state model (model I) for hydrolysis of the scissile phosphodiester connecting nt +1 and −1 is derived from that proposed in (24), according to which the Mg2+ ion at the site termed [B] here directly coordinates to the pro-Rp phosphate oxygen and OH− nucleophile (in green), and simultaneously interacts with the 2′-OH at position −1 via an inner-sphere water molecule. According to model II, two Mg2+ ions, Mg[A] and Mg[B], directly coordinate to the pro-Rp oxygen (7,25), but Mg[A] instead of Mg[B] interacts with the OH− nucleophile via inner-sphere coordination. Additional metal ion interactions at the pro-Sp oxygen (marked Sp; models I and II) and the 3′-bridging oxygen (model I) are conceivable based on strong inhibition effects caused by sulfur substitutions at these positions (7,24,35). The ribose at nt +1 are drawn in the A-helical C3′-endo and the ribose at position −1 in the C2′-endo conformation based on the results of NMR investigations (36,37).