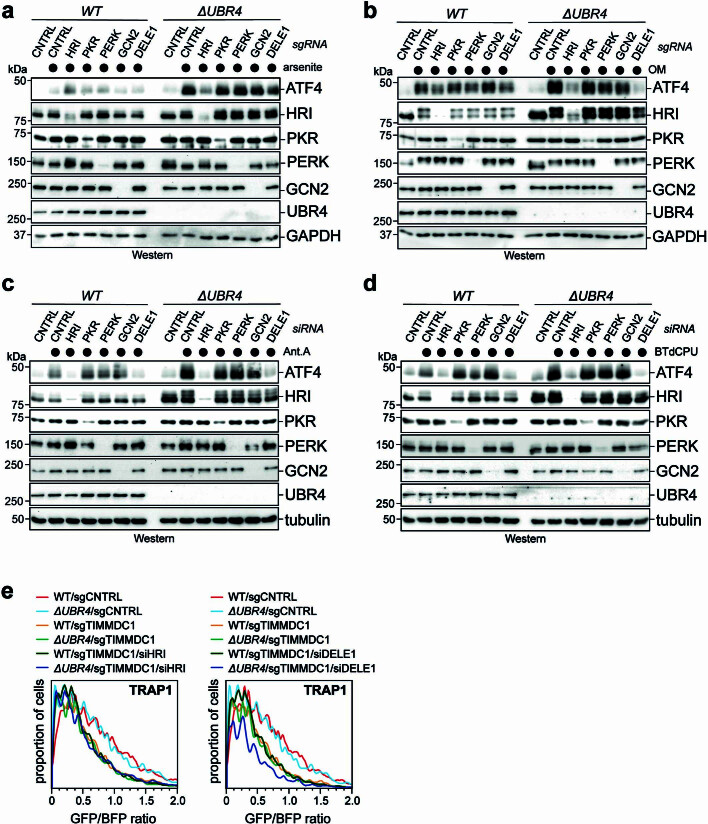
Extended Data Fig. 9. HRI and DELE1 mediate stress response signaling without affecting mitochondrial protein import.
a. Depletion of HRI suppresses increased ISR activation in ΔUBR4 cells treated with 5 μΜ sodium arsenite for 16 h, as monitored by Western blotting using antibodies against ATF4. Similar results in n = 2 independent experiments. b. Depletion of HRI or DELE1 suppresses increased ISR activation in ΔUBR4 cells treated with 25 μΜ oligomycin for 8 h, as monitored by Western blotting using antibodies against ATF4. Similar results in n = 2 independent experiments. c. Depletion of HRI or DELE1 suppresses increased ISR activation in ΔUBR4 cells treated with 0.6 μΜ antimycin A for 16 h, as monitored by Western blotting using antibodies against ATF4. Similar results in n = 2 independent experiments. d. Depletion of HRI or DELE1 suppresses increased ISR activation in ΔUBR4 cells treated with 5 μΜ BTdCPU for 8 h, as monitored by Western blotting using antibodies against ATF4. Similar results in n = 2 independent experiments. e. Depletion of HRI and DELE1 by siRNA does not restore mitochondrial protein import in cells lacking TIMMDC1. Wildtype or ΔUBR4 cells were depleted of TIMMDC1 using specific sgRNAs, as indicated. Mitochondrial protein import was monitored by reconstitution of GFP upon expression of TRAP1-GFP11 co-expressed with BFP in cells stably expressing GFP(1–10) in the mitochondrial matrix. GFP formation upon successful import was monitored by flow cytometry. Experiment was validated using the alternative mitochondrial import substrate HMT2-GFP11. For gel source data, see Supplementary Fig. 1.