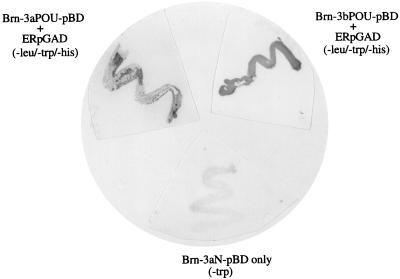
FIG. 4.
Interaction between the POU domains of Brn-3a and Brn-3b with the ER as demonstrated by the yeast two-hybrid system. The POU domains of Brn-3a (Brn-3a POUpBD) and Brn-3b (Brn-3b POUpBD) and the amino terminus of Brn-3a (Brn-3aN pBD) were cloned into the GAL4 DNA-binding domain vector. The full-length cDNA encoding the wild-type ER was cloned into the GAL4 activation domain vector (ER-pGAD). The different Brn-3-containing vectors either were transformed individually or were transformed together with ER-pGAD into competent HF7c yeast and plated out onto agar plates lacking tryptophan only (−trp) or tryptophan, leucine, and histidine (−leu/−trp/−his). Clones which resulted from interaction between the POU domains and ER when grown on agar plates lacking leucine, tryptophan, and histidine were reselected and assayed for activity of the second reporter gene coding for β-galactosidase (top panels). Brn-3aN pBD did not give rise to any clones when cotransformed with ER-pGAD and plated on media lacking leucine, tryptophan, and histidine, but there was growth on agar plates lacking tryptophan. These colonies remained white after the assay for lacZ promoter activity (lower panel).