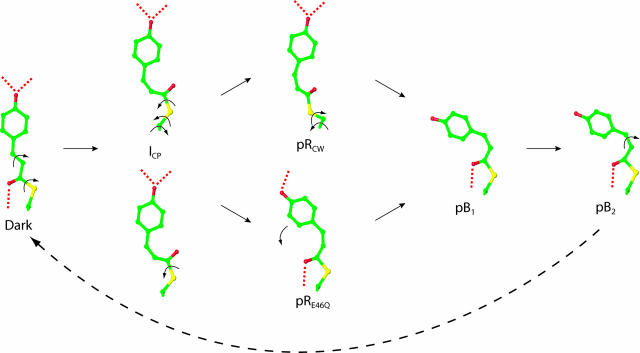
Fig. 3.
Chromophore-binding pocket views of refined intermediate structures and mechanism for the isomerization and rotation of the pCA chromophore upon absorption of blue light. Five distinct structural intermediates (ICP, pRCW, pRE46Q, pB1, and pB2) were identified from four chemical states (α, β, γ, and δ) shown in Fig. 2. ICP is shown twice to demonstrate the biphasic pathways to pRCW and pRE46Q. Isomerization and rotation about single bonds are shown by arrows; hydrogen bonds are dotted. A bicycle pedal mechanism (44), which couples trans-cis isomerization of the C2─C3 double bond with rotation about a nonadjacent single bond, is used for the dark state to ICP transition. Further rotations about single bonds result in the pB1 conformation. pB2 reverts thermally to the dark state with no further detectable intermediates.