Figure 1.
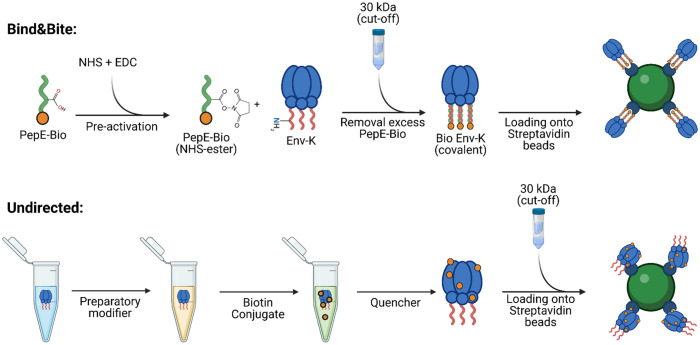
Biotinylation reactions for oriented and undirected display of trimers of HIV Env. To investigate the impact of antigen orientation on IgG recognition, antigen-specific B cell activation, and phagocytic uptake, the same HIV Env protein was biotinylated either at the C-terminus by a recently developed site-selective covalent coupling strategy named “Bind&Bite” (upper panel) or, in an unselective fashion, at free amines (lower panel). The Bind&Bite method involves site-directed modifications of proteins through the formation of heterodimeric coiled coils. An acidic 21 amino acid peptide with a biotin molecule attached to its C-terminus (PepE-Bio) is pre-activated with EDC and NHS, resulting in the formation of NHS esters at glutamate side chains. Subsequently, the activated PepE-Bio is incubated at a 2-fold molar excess with Env-K (HIV-1 BG505 gp140 Env trimer with the complementary Peptide K fused in frame to the C-terminus), followed by the removal of unbound PepE-Bio via dialysis. The purified and concentrated Env-K-Bio is then loaded onto streptavidin-coated beads for further functional analyses. Biotinylation randomly at free amines was performed with a commercial biotin conjugation kit (Abcam). Env-K is treated with the provided “Modifier” reagent to add sulfhydryl groups at free amines. The antigen is then incubated with the biotin conjugate and remaining free labels have their reactive groups blocked via the “Quencher” reagent. Lastly, unbound biotin and buffer is exchanged to PBS through dialysis. PepE-Bio: biotinylated Peptide E; EDC: 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide; NHS: N-Hydroxysuccinimide. Created with BioRender.com.