Figure 1.
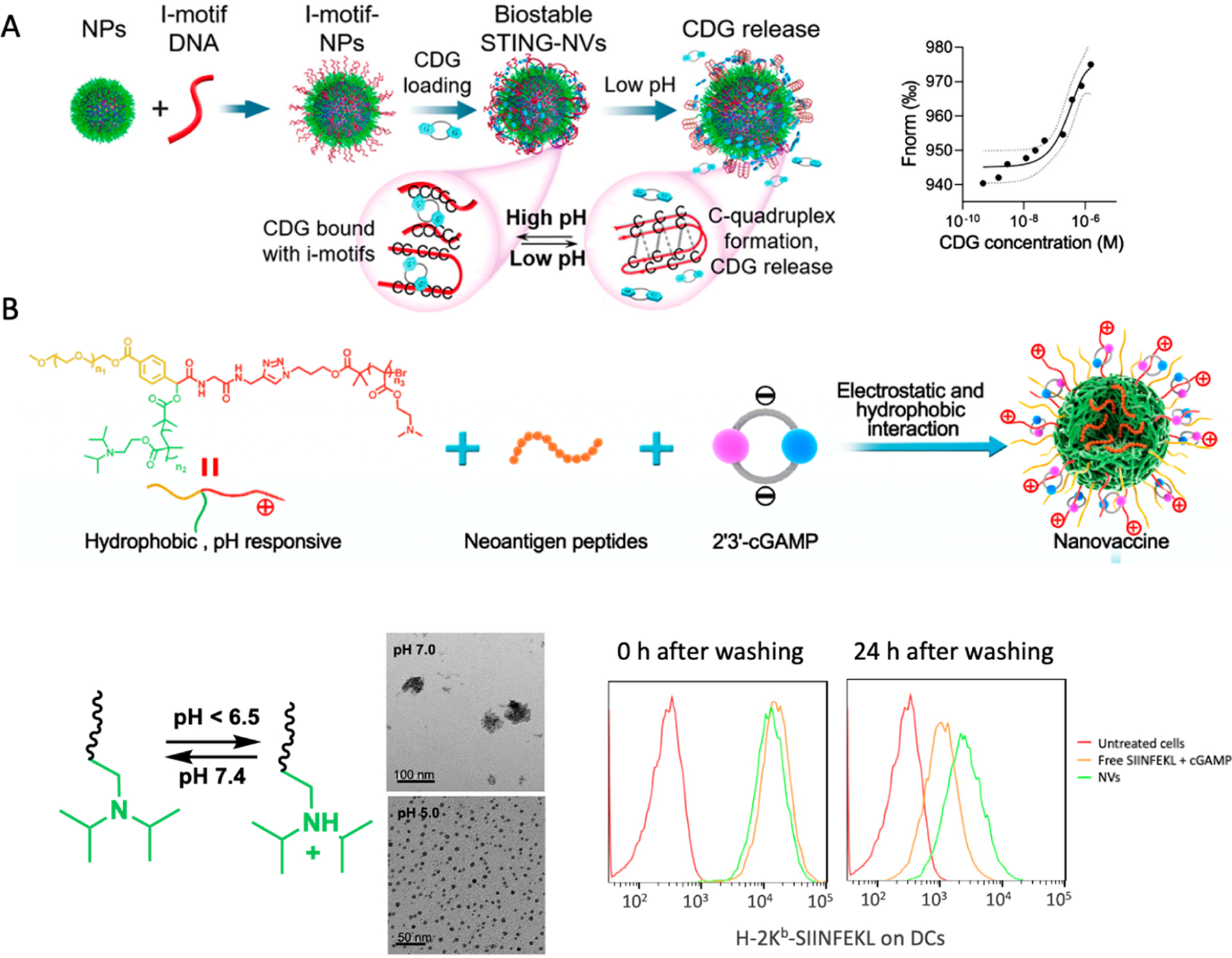
Development of STING agonist-based NVs for cancer immunotherapy. (A) Design of STING-NVs based on pH-responsive DNA i-motif-functionalized PEG-b-PLA NPs for CDG loading via hydrogen bonding. These STING-NVs efficiently loaded CDG by hydrogen bonding (i.e., G:C base pairing). Under acidic conditions, CDG was dissociated from i-motifs due to the formation of C-quadruplexes from i-motifs through protonated C:C+ base-pair formation. As the driving force of CDG loading in these NPs, the binding affinity (Kd) of CDG with NPs was determined to be 47 × 10−9 M, as measured by microscale thermophoresis (MST). (B) pH-responsive polymeric NVs for the codelivery of cGAMP and tumor neoantigen peptides through electrostatic and hydrophobic interactions, respectively. The pH-responsive PDPA moiety was protonated at acidic environment, such as endolysosome inside APCs, to induce NV dissociation and vaccine release from NPs. The codelivery of cGAMP and SIINFEKL peptide sustained the antigen presentation even after washing off extracellular vaccines as shown by flow cytometric analysis using a dye-labeled antibody for the SIINFEKL/H-2Kb complexes (H-2Kb is a subtype of murine MHC-I). The NVs potentiated the neoantigen-specific T cell responses, resulting in robust tumor immunotherapy. Adapted with permission from refs 1 and 59. Copyright 2022 for ref 1, 2020 for ref 59 by Wiley-VCH GmbH.
