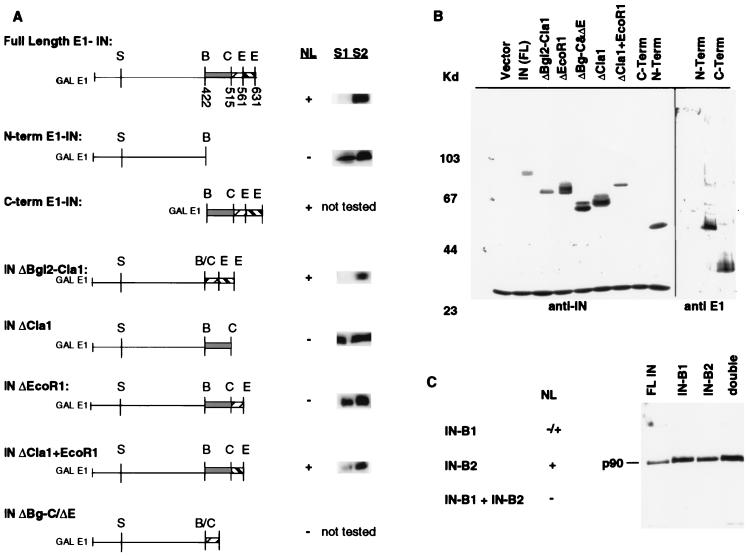
FIG. 3.
Analysis of E1-IN fusion proteins. (A) C-terminal deletion constructs map the NLS region within the last 74 amino acids of IN. Constructs tested are diagrammed, with restriction sites indicated; abbreviations for these are defined in the legend to Fig. 1. Numbers shown below restriction sites in full-length E1-IN represent amino acid residues. Nuclear localization (NL) is indicated as present (+) or absent (−); these results are from the indirect immunofluorescence experiments with anti-IN antibody described in the legend to Fig. 2, except for that for the C-terminal construct that necessitated the use of anti-E1 (see text). Cell fractionation experiments (described in Materials and Methods) were performed on strains bearing the E1-IN truncation constructs. Bands corresponding to the indicated E1-IN protein are shown to the right of the NL data. (B) Immunoblot analysis of the truncated IN proteins. Whole-cell extracts were electrophoresed on an SDS–8% polyacrylamide gel. Proteins were detected with either MAb 8B11 (1:5,000) or anti-E1 (1:500). Molecular mass markers are shown at the left in kilodaltons. MAb 8B11 also recognizes a non-Ty1 band of ∼30 kDa. Since the C-terminal truncation does not contain the epitope for MAb 8B11, this protein extract, along with the N-terminal one, was probed with anti-E1. (C) Similar immunoblot analysis of the substitution mutant GAL-E1-IN constructs B1 (NSKKRS→AAGSAA) and B2 (RSKKRI→AAGSAA). NL is indicated as present (+), partial (−/+), or absent (−).