Abstract
The Reward Positivity (ΔRewP) event-related potential (ERP), generally quantified as the difference between neural responsiveness to monetary gains (RewP-Gain) versus losses (RewP-Loss) is commonly used as an index of neural reward responsiveness. Despite the popularity of this ERP component in studies of reward processing, knowledge about the role of state-related influences on the ΔRewP is limited. The present study examined whether ΔRewP amplitudes may differ based on when during the day they are assessed and whether age or gender would moderate this link. Participants were 188 children between the ages of 7 and 11 (47.3% female) without a lifetime history of DSM-IV major depressive disorder or any anxiety disorder recruited from the community. Children completed the Doors task during which continuous electroencephalography was recorded to isolate the ΔRewP. To better isolate this ERP component from other temporally or spatially overlapping ERPs, we used temporospatial principal component analysis. We found that time of day (ToD) differences in the ΔRewP amplitude varied based on children’s age. Specifically, older, compared to younger, children exhibited stronger responses to gains versus losses between 11:15 am and 12:30 pm and after around 5:15 pm. Further, these age-related differences appeared to be driven specifically by older children’s reduced neural responsiveness to losses. The findings have methodological implications by highlighting the importance of accounting for the ToD at which ΔRewP-focused study sessions are conducted as well as for demographic characteristics of the participants, such as their age.
Keywords: rewards, ERP, reward positivity, children, time of day
1. Introduction
Reward processing is multidimensional (Berridge & Kringelbach, 2008) and plays a crucial role not only in normative functioning but also in various forms of psychopathology (e.g., Nusslock & Alloy, 2017; Webb, 2017). To quantify individual differences in reward processing, a number of researchers have focused on the Reward Positivity (ΔRewP; also referred to in the literature as the feedback negativity [FN] and feedback-related negativity [FRN]) event-related potential (ERP) component. Often quantified as the difference between neural responsiveness to monetary gains (RewP-Gain) versus losses (RewP-Loss), the ΔRewP is maximal over frontocentral recording sites and peaks approximately 250–300 ms after receiving gain or loss feedback (for reviews, see Glazer, Kelley, Pornpattananangkul, Mittal, & Nusslock, 2018; Proudfit, 2015). This ERP component is thought to be sensitive to both performance and feedback evaluation and has been utilized as an index of neural reward-related feedback responsiveness in a large number of studies (for reviews, see Glazer et al., 2018; Proudfit, 2015).
Despite the popularity of the ΔRewP in investigations of reward responsiveness, little is known about the potentially important role of state-related influences on this ERP component. For example, researchers examining reward responsiveness rarely, if ever, take into account the time of day (ToD) at which the participant completed the study. This constitutes a significant gap in the literature given clear evidence of an intrinsic 24-hour rhythmicity in human biology coordinated by the circadian system (for a review, see Mohawk, Green, & Takahashi, 2012), which likely has an impact on the ΔRewP. In line with this possibility, there is evidence for diurnal variation in positive affect, with the peak in positive states observed between midday and early evening and a decline in these states starting around 9 pm (e.g., Clark, Watson, & Leeka, 1989; Murray, Allen, & Trinder, 2002; Watson, Wiese, Vaidya, & Tellegen, 1999). In addition, a recent review of human fMRI research found evidence for circadian modulation of neural activation in brain regions associated with the anticipation and receipt of rewards (Byrne et al., 2019). However, although indicative of an important link between human circadian system and neural reward-related activation, it is unclear whether these fMRI findings would generalize to ERPs, such as the ΔRewP.
The present study aimed to address this gap in the literature by evaluating whether there are ToD differences in ΔRewP amplitude in a large community sample of children. Although the present study did not specifically assess circadian rhythms, a recent review of reward-related circadian modulation of neural activation provides convincing evidence for the usefulness of examining ToD in this line of inquiry (Byrne et al., 2019). Indeed, even if the ToD effects are not strictly circadian, they have important methodological implications with regard to participant scheduling and accounting for potential confounds. Further, because ours is the first study to examine the ToD effects on the ΔRewP, we focused only on healthy controls to avoid any influence of psychopathology on reward functioning (cf. Nusslock & Alloy, 2017; Webb, 2017). Relatedly, specifically focusing on children prior to the potential onset of depression and/or anxiety in these children constitutes an important effort in early identification and prevention of psychopathology. Finally, to mimic prior studies that focused on the ΔRewP, thereby maximizing the generalizability of our findings to such studies, participants were not randomly assigned to different ToDs but rather were free to choose which of the available time slots to sign up for. In line with prior research demonstrating the peak in positive states between midday and early evening (e.g., Clark et al., 1989; Murray et al., 2002; Watson et al., 1999), we expected that the strongest differentiation between gains versus losses (i.e., larger ΔRewP) would be observed in the mid-to-late afternoon. Therefore, we examined both linear and nonlinear (quadratic and cubic) ToD effects on ΔRewP amplitude. Because a number of studies demonstrate the presence of age- and/or gender-related differences in neural correlates of feedback processing (e.g., Crowley et al., 2013; Ding et al., 2017; Kujawa et al., 2013; Vrticka et al., 2014), we also examined whether children’s age and/or gender would moderate the link between ToD and the ΔRewP amplitude. Due to the exploratory nature of these moderation analyses, no specific hypotheses were made.
2. Method
2.1. Participants
Participants were 188 children recruited from the community, who were a subset of children participating in a larger study examining correlates of depression and anxiety in children. To be eligible to participate in the larger study, children had to be between the ages of 7 and 11 years old and have no learning or developmental disorders that would make it difficult for them to complete the study. For the current study, we excluded any children with a lifetime history of DSM-IV major depressive disorder (MDD) or any anxiety disorder to avoid the potential influence of psychopathology on reward functioning. The average age of the children in our study was 9.73 years (SD = 1.41) and 47.3% were female. In terms of race, 72.9% of the children were Caucasian, 12.8% were African American, 12.8% were biracial, and 1.5% were from other racial groups. In terms of ethnicity, 8.5% of the children were Hispanic.
2.2. Measures
2.2.1. Diagnoses and Symptoms.
The Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) was used to assess for current and past DSM-IV diagnoses of MDD and anxiety disorders in children. To assess interrater reliability, a subset of 20 diagnostic interviews from this project was coded by a second interviewer and kappa coefficients for diagnoses of MDD and anxiety disorders were good (all κ ≥ .86).
2.2.2. Reward task.
The reward task was a simple guessing Doors task that is commonly used in studies of reward processing (e.g., Bress, Smith, Foti, Klein, & Hajcak, 2012; Bress, Meyer, & Hajcak, 2015; Foti, Weinberg, Dien, & Hajcak, 2011; Kujawa, Proudfit, & Klein, 2014; Nelson, Perlman, Klein, Kotov, & Hajcak, 2016; Tsypes, Owens, Hajcak, & Gibb, 2018). The task consisted of 50 trials, presented in 2 blocks of 25 trials. Participants were shown an image of two doors at the beginning of each trial and instructed to guess which door had a monetary prize behind it by pressing either the left or right button on a game controller. They were informed that, on each trial, they could either win $0.50, as indicated by a green up-arrow, or lose $0.25, as indicated by a red down-arrow. Feedback about having chosen correctly or incorrectly was presented for 2,000 ms and then followed by the message “Click for the next round.” This message remained on the screen until the participant responded and the next trial began. Across the task, 25 gain and 25 loss trials were presented in a random order.
2.2.3. Time of Day.
ToD was quantified as the time when participants completed the Doors task. It was recorded in military (24 hour) time and then minutes were converted to reflect fractions of hours. In this study, the time at which children completed the Doors task ranged from 9.67 to 18.93 hours. See Tables 1 and 2 for a more detailed information about the distribution of participants across hours of the day. Please note, however, that ToD was treated as a continuous variable in all analyses.
Table 1.
Distribution of The Time of Day Bins by Child Age and Gender
| 9–10 | 10–11 | 11–12 | 12–1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | 6–7 | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Age 7 | 1 | 3 | 5 | 1 | 2 | 3 | 1 | 2 | 6 | 5 |
| Age 8 | 1 | 7 | 1 | 2 | 0 | 3 | 3 | 3 | 6 | 3 |
| Age 9 | 2 | 5 | 8 | 4 | 2 | 1 | 2 | 5 | 7 | 5 |
| Age 10 | 2 | 12 | 5 | 3 | 2 | 2 | 4 | 4 | 10 | 3 |
| Age 11 | 1 | 10 | 7 | 1 | 2 | 2 | 2 | 8 | 7 | 2 |
| Total: | 7 | 37 | 26 | 11 | 8 | 11 | 12 | 22 | 36 | 18 |
| Boys | 6 | 20 | 13 | 6 | 5 | 4 | 5 | 10 | 20 | 10 |
| Girls | 1 | 17 | 13 | 5 | 3 | 7 | 7 | 12 | 16 | 8 |
Although we list data in “bins” to facilitate presentation, time of day and child age were treated as continuous variables in all analyses.
Table 2.
Means and Standard Deviations of the RewP Variables by Time of Day
| 9–10 | 10–11 | 11–12 | 12–1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | 6–7 | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| RewP-Gain M (SD) | 10.13 (5.67) | 6.25 (9.32) | 6.18 (6.46) | 5.16 (6.13) | 6.92 (7.74) | 6.35 (4.82) | 6.59 (5.05) | 8.09 (5.65) | 8.16 (7.15) | 6.60 (7.39) |
| RewP-Loss M (SD) | 6.82 (7.73) | 3.17 (7.60) | 4.56 (7.13) | 2.36 (4.15) | 3.41 (11.47) | 4.84 (6.75) | 2.90 (8.16) | 4.02 (6.27) | 5.43 (6.64) | 3.75 (10.57) |
| ΔRewP M (SD) | 3.31 (5.02) | 3.08 (7.29) | 1.62 (6.08) | 2.80 (6.55) | 3.51 (7.28) | 5.77 (1.51) | 3.69 (7.07) | 4.07 (6.93) | 2.73 (7.87) | 2.85 (10.06) |
Note. Although we list data in “bins” to facilitate presentation, time of day was treated as a continuous variable in all analyses.
2.2.4. EEG data acquisition and processing.
During the task, continuous EEG was recorded using a custom cap and the BioSemi ActiveTwo system. The EEG was digitized at 24-bit resolution with a sampling rate of 512 Hz. Recordings were taken from 34 scalp electrodes based on the 10/20 system. The electrooculogram was recorded from four facial electrodes. Off-line analysis was performed using the Matlab extension EEGLAB (Delorme & Makeig, 2004) and the EEGLAB plug-in ERPLAB (Lopez-Calderon & Luck, 2010). All data were re-referenced to the average of the left and right mastoid electrodes and band-pass filtered with cutoffs of 0.1 Hz and 30 Hz. EEG data were processed using both artifact rejection and correction. Large and stereotypical ocular components were identified and removed using independent component analysis (ICA) scalp maps (Jung et al., 2001). Epochs with large artifacts (greater than 100μV) were excluded from analysis. EEG was segmented for each trial, beginning 200 ms before onset of the feedback stimulus and ending 1000 ms after onset of the feedback stimulus. Based on the findings of a recent comprehensive study on internal consistency of fMRI and EEG measures of reward in late childhood and early adolescence demonstrating that internally consistent measure of response to gain and loss can be obtained using just 14 gain and 14 loss trials of the Doors task (Luking, Nelson, Infantolino, Sauder, & Hajcak, 2017), we focused only on children who had at least 14 trials per condition. In our sample, the average number of gain trials remaining following artifact rejection was 23.28 (SD = 2.03; range 16–25) and the average number of loss trials was 23.04 (SD = 2.39; range 15–25). ERPs were separately averaged across gain and loss trials, and the activity 200 ms before feedback onset served as the baseline. These averages were then exported for temporospatial principal component analysis (PCA), which allows for the isolation of the RewP from the overlapping components. The key strength of PCA, therefore, is that it allows the isolation of the RewP from other temporally or spatially overlapping components, allowing a specific assessment of neural reactivity to gains and losses. The PCA was conducted using the ERP PCA Toolkit, version 2.69 (Dien, 2010a). Consistent with the published guidelines for the use of PCA with ERP data (Dien, 2010b), a temporal Promax rotation was performed first to rotate to simple structure in the temporal domain. The temporal PCA used all time points as variables, including all participants, two conditions (i.e., gains and losses), and 34 recording sites as observations. Based on a parallel test (Horn, 1965), ten temporal factors were extracted for rotation, which accounted for 95.0% variance in the ERP signal. Following the temporal PCA, a spatial Infomax rotation was performed on each temporal factor to reduce the spatial dimensions of the datasets. The spatial PCA used recording sites as variables and all participants, conditions, and temporal factor scores as observations. Based on a parallel test (Horn, 1965), three spatial factors were extracted from each temporal factor, which resulted in a total of 30 temporospatial factor combinations. To facilitate interpretation of the PCA solutions, after analysis, the ERP PCA Toolkit automatically reproduces the original data, re-creating the waveform in microvolts of each factor loading by multiplying the correlation factor loadings with the standard deviations of the variables (Dien, 2006). The toolkit then reports the peak channel and peak time point for each factor (Dien, 2010a,b). The PCA-derived factor TF5SF1, which accounted for 2.4% of total variance and resembled the RewP in its temporal and spatial distribution was used in the statistical analyses reported below.
2.3. Procedure
Upon arrival at the laboratory, parents were asked to provide informed consent and children were asked to provide assent to be in the study. Next, the child completed the Doors task. During this time, the K-SADS-PL was administered to the parent by a trained interviewer. Following this, the same interviewer who had administered the K-SADS-PL to the parent also administered it to the child. The Institutional Review Board approved all procedures. Families were compensated a total of $80 for their participation in the larger study and children received a $10 gift card to a local store. All children also received a bonus of $5 for completing the Doors task.
3. Results
First, we examined the effect of ToD on the RewP using general linear models with ToD, condition (gains, losses), and the ToD × Condition interaction as predictors and RewP amplitude serving as the dependent variable. We examined linear, quadratic, and cubic ToD effects in separate models, with the quadratic model also including the linear trend as a covariate and the cubic model including the linear and quadratic trends as covariates. Although the main effect of condition was significant in each of the models (all ps < .05), with larger responses for RewP-Gain than for RewP-Loss, the main effect of ToD and the ToD × Condition interaction were not significant in any of these analyses (lowest p = .14).
Second, we tested for potential age differences in the ToD effects by adding child age to the models (Note: Child age was treated as a continuous variable in all analyses with exact age denoted by fractions of years). For the linear ToD effects model, there was a significant main effect of condition on the RewP amplitude, F(1,184) = 33.41, p < .001, ηp2 = .15, as well as a significant Age × Condition interaction, F(1,184) = 12.74, p < .001, ηp2 = .07. Follow-up analyses revealed that whereas RewP-Gain was positively correlated with child age, r = .16, p = .03, the correlation between RewP-Loss and child age was not significant, r = −.09, p = .22. No other main effects or interactions were significant (lowest p = .06). For the quadratic ToD effects model, none of the main or interaction effects with ToDQuadratic were significant (lowest p = .29). For the cubic ToD effects model, there was a significant main effect of condition, F(1,180) = 6.01, p = .02, ηp2 = .03, as well as significant Age × ToDLinear, F(1,180) = 4.21, p = .04, ηp2 = .02, Age × ToDCubic F(1,180) = 4.98, p = .03, ηp2 = .03, and Age × ToD × Condition, F(1,180) = 4.48, p = .04, ηp2 = .02, interactions (for more details, see Table 1 in the supplement).
Given the significant Age × ToD × Condition interaction, we conducted follow-up tests to determine regions of significance across ToD using the ESTIMATE statement in SAS. Focusing first on the ΔRewP difference score (reflecting the difference in neural response to gains versus losses) as the outcome variable, we entered age, the three ToD variables (linear, quadratic, cubic), and the three Age × ToD interactions into the model. The Age × ToDCubic interaction was significant, t(180) = 2.12, p = .04, and the regions of significance tests indicated that significant main effect of age on the ΔRewP first emerged around 11:15 am, remained significant until around 12:30 pm, and then re-emerged around 5:15 pm. In both of these time windows, older children exhibited significantly stronger responses to gains vs. losses than younger children. Indeed, by the last time window (6:00–7:00pm), younger children exhibited stronger responses to losses than gains (negative ΔRewP score). These results are depicted in Figure 1, solving the regression equations for values 1 SD above and below the mean for child age. To further explore the Age × ToDCubic × Condition interaction, we also examined the effects of the Age × ToDCubic interaction on RewP-Loss and RewP-Gain amplitudes, separately. The Age × ToDCubic interaction was significant for RewP-Loss amplitudes, t(180) = −2.90, p = .004 but not for RewP-Gain amplitudes, t(180) = −0.96, p = .34 (for more details, see Tables 2–4 in the supplement). The pattern of the RewP-Loss effects was similar to that observed for ΔRewP, with the strongest age-related differences emerging later in the day. Specifically, starting around 5:45 pm, younger children exhibited significantly larger RewP-Loss magnitudes than older children (see Figure 2).
Figure 1.
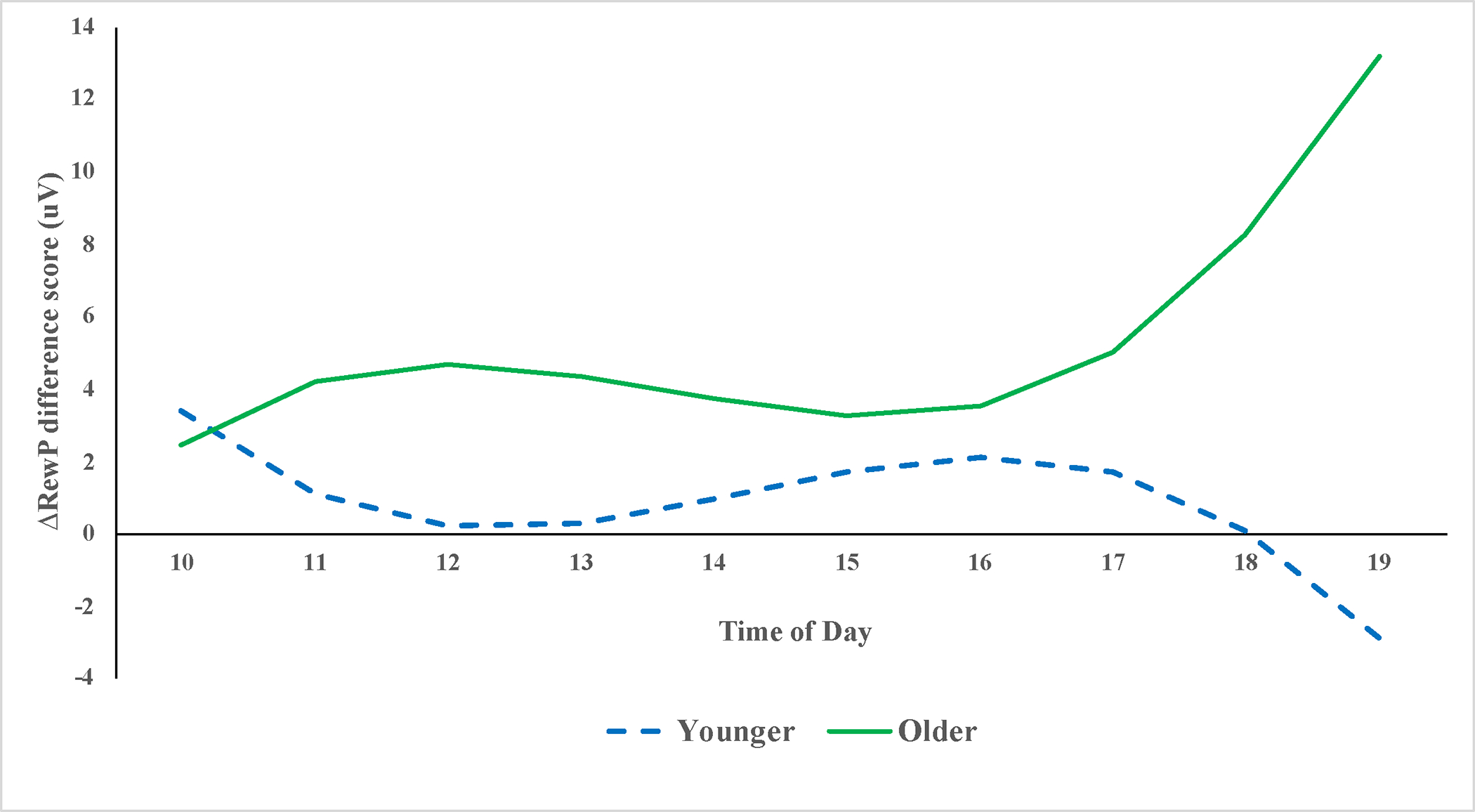
Time of Day Differences in ΔRewP Amplitude for Younger Versus Older Children.
Figure 2.
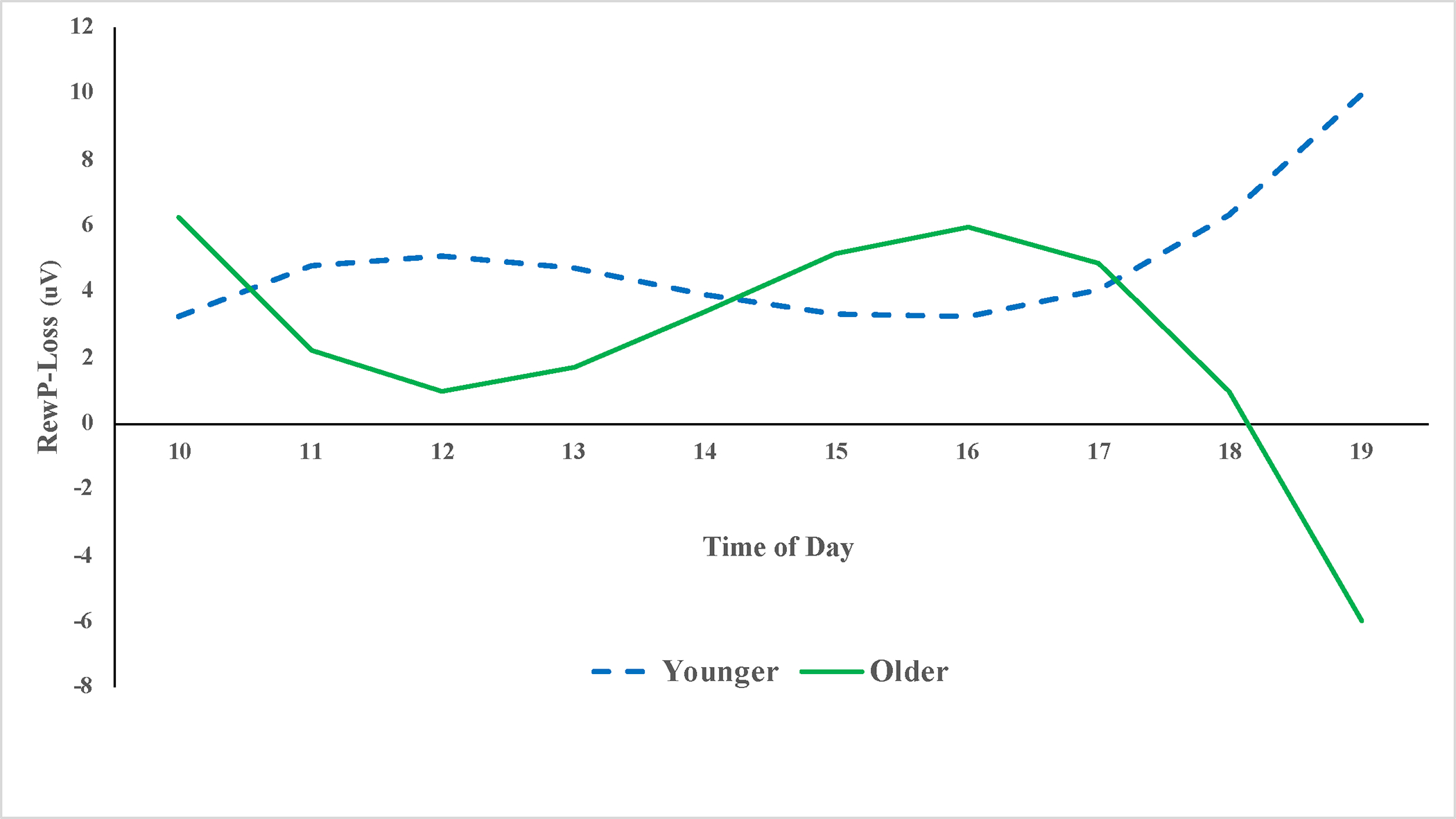
Time of Day Differences in RewP-Loss Amplitude for Younger Versus Older Children.
Finally, we tested for gender differences in the ToD effects. Although the main effect of condition was significant in each of the models (all ps < .05), with larger responses for RewP-Gain than for RewP-Loss, none of the other main effects or interactions reached statistical significance (lowest p = .06)1,2.
4. Discussion
The primary goals of this study were to examine the effects of ToD on the ΔRewP amplitude in a large sample of children with no lifetime history of MDD or anxiety and to determine whether these effects would be moderated by children’s age and/or gender. We expected to observe the strongest differentiation between gains versus losses (i.e., larger ΔRewP) in the mid-to-late afternoon. Contrary to our expectations, ToD differences in the ΔRewP amplitude only emerged in exploratory analyses that also considered children’s age. Specifically, older, compared to younger, children exhibited stronger responses to gains versus losses, indicating heightened overall reward responsiveness, between 11:15 am and 12:30 pm and after around 5:15 pm. Further, these age-related differences appeared to be driven specifically by older children’s reduced neural responsiveness to losses. These findings are consistent with the well-documented normative increases in reward responsiveness and attenuated reactivity to aversive stimuli as children transition into adolescence (for a review, see Spear, 2011). These reward functioning-related changes in adolescence are paralleled by sleep and circadian shifts, including eveningness, defined as higher preference for later sleep times (Crowley, Acebo, & Carskadon, 2007). Thus, the present study provides important preliminary evidence for the need to account for these developmental changes in the studies of reward processing by also considering ToD.
Conclusions must remain tentative pending replication of these findings. However, they suggest that studies of reward processing need to consider the time at which youth are assessed to ensure that conclusions about the links between the RewP and some outcome are being driven by ToD differences in reward responsiveness. Specifically, to the extent that certain outcomes (e.g., depression) are correlated with child age (e.g., risk for depression increases as children age into adolescence; for reviews, see Gibb, 2014; Rudolph & Flynn, 2014), then researchers may need to be cautious in conducting their studies in the late afternoon/early evening, which is common in studies with pediatric samples where assessments are often conducted after school hours. Another potential implication is that studies examining age-related changes in RewP amplitude (e.g., Kujawa et al., 2018) may need to take ToD effects into account. Otherwise, developmental differences could be obscured. A strength of the study was the use of PCA, which allows for the isolation of the ΔRewP from the overlapping ERP components and thus minimizes inconsistencies in some prior studies that relied on window-based approaches to quantify the ΔRewP (for further discussion of the issue, see Glazer et al., 2018).
Despite the contributions of the present study to the currently limited knowledge on the influences of state- and participant-related characteristics on the ΔRewP, the study had several limitations that represent useful directions for future empirical investigations. First, it will be important to assess additional relevant variables with a circadian rhythm (e.g., cortisol), in order to better distinguish reward-related processes from other cyclic processes that occur within the human nervous system. It will also be important to understand circadian functioning beyond the hours examined in the present study (e.g., night time, early morning). Relatedly, the study included fewer data points during certain ToDs and the statistical estimates might have been more reliable with a greater number of observations in such cases. Thus, future studies might seek to randomize participants to ToD to minimize the potential influence of factors that may covary with ToD preference as well as specifically recruit an equivalent number of participants throughout the day. Second, the present study focused only on healthy children between ages of 7 and 11 and thus investigations of the generalizability of our findings to other age groups and degree of psychiatric impairment are warranted. Third, because the present study focused specifically on the RewP, future studies should also examine the potential effects of ToD on other reward-related EEG/ERP components (as reviewed in Glazer et al., 2018). Fourth, whereas the present study only focused on one type of task and one stimulus type (i.e., monetary losses and gains), it will be important for future research to examine whether our findings would generalize to other experimental paradigms and reward stimuli. Finally, because the current investigation only focused on one aspect of reward processing (i.e., liking/Initial Response to Reward subconstruct within the RDoC Positive Valence Systems domain), additional research into the role of circadian modulation on other aspects of reward processing is needed.
In summary, our findings provide novel insights into the understudied yet important influences of state- and participant-related characteristics on the ΔRewP. Specifically, they have methodological implications by suggesting the importance of assessing and accounting for the ToD at which the ΔRewP-focused study sessions are conducted. In addition, these age-related results are in line with the recently underscored need for the inclusion of demographic variables as moderators as a standard practice in all psychophysiological studies, in order to fully understand their impact on the EEG/ERP variables of interest (Hill, Oumeziane, Novak, Rollock, & Foti, 2018).
Supplementary Material
Acknowledgements
The project was supported by the National Research Service Award MH114319 from the National Institute of Mental Health awarded to Aliona Tsypes and the National Institute of Mental Health grant MH098060 awarded to Brandon Gibb. We would like to thank Katie Burkhouse, Mary Woody, Anastacia Kudinova, Cope Feurer, Sydney Meadows, Michael Van Wie, Devra Alper, Eric Funk, Effua Sosoo, Nathan Hall, Kiera James, Aholibama Lopez, Max Owens, and Kristina Wong for their help in conducting assessments for this project. We would also like to thank John Merranko for his help with the SAS analyses. The authors have no conflicts of interest to disclose.
Footnotes
In addition to the ToD effects, we also examined the potential effects of the day length on the ΔRewP as well as potential age and gender differences in these effects. None of the main effects or interactions with day length were significant.
We also examined several potentially relevant correlations. First, neither age nor sex were significantly correlated with the ToD at which the Doors task was completed (lowest p = .11). Second, neither the time of year (i.e., day number consecutively from January 1 each year) nor whether school was in session (coded dichotomously into school year versus summer) were significantly corelated with any of the RewP variables (lowest p = .26).
References
- Berridge KC, & Kringelbach ML (2008). Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology, 199, 457–480. 10.1007/s00213-008-1099-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Meyer A, & Hajcak G (2015). Differentiating anxiety and depression in children and adolescents: Evidence from event-related brain potentials. Journal of Clinical Child and Adolescent Psychology, 44, 238–249. 10.1080/15374416.2013.814544 [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, & Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology, 89, 156–162. https://doi.org/j.biopsycho.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JEM, Tremain H, Leitan ND, Keating C, Johnson SL, & Murray G (2019). Circadian modulation of human reward function: Is there an evidentiary signal in existing neuroimaging studies? Neuroscience and Biobehavioral Reviews, 99, 251–274. 10.1016/j.neubiorev.2019.01.025 [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, & Leeka J (1989). Diurnal variation in the positive affects. Motivation and Emotion, 13, 205–234. 10.1007/BF00995536 [DOI] [Google Scholar]
- Crowley SJ, Acebo C, & Carskadon MA (2007). Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine, 8, 602–612. 10.1016/j.sleep.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Hommer RE, South M, Molfese PJ, Fearon RM, & Mayes LC (2013). A developmental study of the feedback-related negativity from 10–17 years: Age and sex effects for reward versus non-reward. Developmental Neuropsychology, 38, 595–612. 10.1080/87565641.2012.694512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods, 134, 9–21. 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ding Y, Wang E, Zou Y, Song Y, Xiao X, Huang W, & Liu Y (2017). Gender differences in reward and punishment for monetary and social feedback in children: An ERP study. PLoS ONE, 12, e0174100. 10.1371/journal.pone.0174100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J (2006). Progressing towards a consensus on PCA of ERPs. Clinical Neurophysiology, 117, 695–707. 10.1016/j.clinph.2005.09.029 [DOI] [PubMed] [Google Scholar]
- Dien J (2010a). The ERP, PCA toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods, 187, 138–145. 10.1016/j.jneumeth.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Dien J (2010b). Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology, 47, 170–183. 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, & Hajcak G (2011). Event-related potential activity in the basal ganglia differentiates rewards from non-rewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping, 32, 2207–2216. 10.1002/hbm.21182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE (2014). Depression in children. In Gotlib IH & Hammen CL (Eds.), Handbook of depression. (pp. 374–390). New York: Guilford. [Google Scholar]
- Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal., & V.A., Nusslock R (2018). Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. International Journal of Psychophysiology, 132, 184–202. 10.1016/j.ijpsycho.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Hill KE, Oumeziane BA, Novak KD, Rollock D, & Foti D (2018). Variation in reward- and error-related neural measures attributable to age, gender, race, and ethnicity. International Journal of Psychophysiology, 132, 353–364. 10.1016/j.ijpsycho.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Horn JL (1965). A rationale and test for the number of factors in factor analysis. Psychometrika, 30, 179–185. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, McKeown MJ, Bell AJ, Lee TW, & Sejnowski TJ (2001). Imaging brain dynamics using independent component analysis. Proceedings of the IEEE, 89, 1107–1122. 10.1109/5.939827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children - present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of American Academy of Child and Adolescent Psychiatry, 36, 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Carroll A,Mumper E,Mukherjee D, Kessel EM, Olino T, ... & Klein DN (2018). A longitudinal examination of event-relate potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. International Journal of Psychophysiology, 132, 323–330. 10.1016/j.ijpsycho.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123, 287–297. 10.1037/a0036285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Smith E, Luhmann C, & Hajcak G (2013). The feedback negativity reflects favorable compared to nonfavorable outcomes based on global, not local, alternatives. Psychophysiology, 50, 134–138. 10.1111/psyp.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8. 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Nelson BD, Infantolino ZP, Sauder CL, & Hajcak G (2017). Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biological Psychiatry, 2, 289–297. 10.1016/j.bpsc.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, & Takahashi JS (2012). Central and peripheral circadian clocks in mammals. Annual Review of Neuroscience, 35, 445–462. 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Allen NB, & Trinder J (2002). Mood and the circadian system: Investigation of a circadian component in positive affect. Chronobiology International, 19, 1151–1169. 10.1081/CBI-120015956 [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted neural response to rewards prospectively predicts the development of depression in adolescent girls. American Journal of Psychiatry, 173, 1223–1230. 10.1176/appi.ajp.2016.15121524 [DOI] [PubMed] [Google Scholar]
- Nusslock R & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. 10.1016/j.jad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52, 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, & Flynn M (2014). Depression in adolescents. In Gotlib IH & Hammen CL (Eds.), Handbook of depression. (3rd ed., pp. 391–409). New York: Guilford. [Google Scholar]
- Spear LP (2011). Reward, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience, 1, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsypes A, Owens M, Hajcak G, & Gibb BE (2018). Neural reward responsiveness in children who engage in nonsuicidal self-injury: an ERP study. Journal of Child Psychology and Psychiatry, 59, 1289–1297. 10.1111/jcpp.12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P, Sander D, Anderson B, Badoud D, Eliez S, & Debbané M (2014). Social feedback processing from early to late adolescence: Influence of sex, age, and attachment style. Brain and Behavior, 4, 703–720. 10.1002/brb3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, & Tellegen A (1999). The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology, 76, 820–838. 10.1037/0022-3514.76.5.820 [DOI] [Google Scholar]
- Webb IC (2017). Circadian rhythms and substance abuse: Chronobiological considerations for the treatment of addiction. Current Psychiatry Reports, 19, 1–12. 10.1007/s11920-017-0764-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


