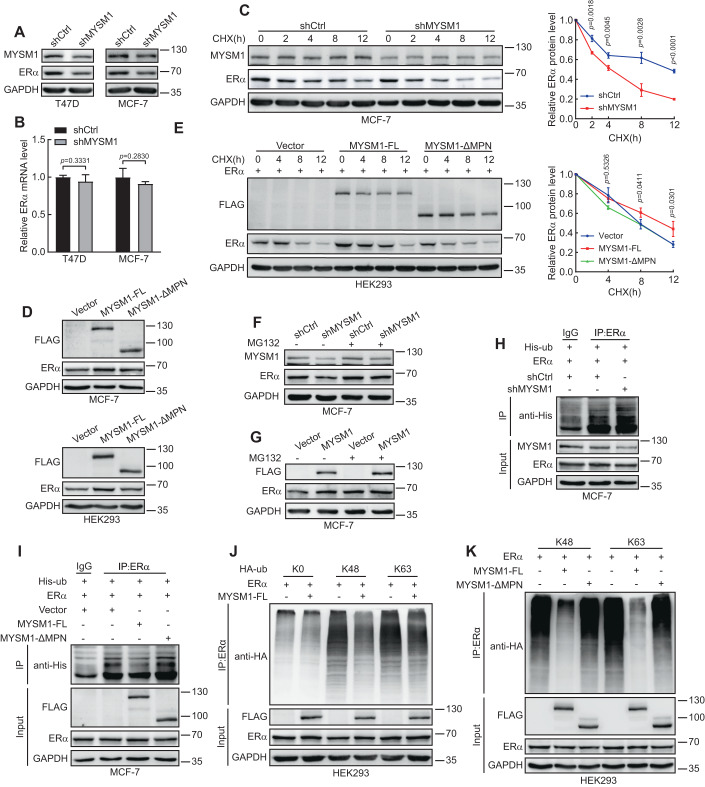
Figure 3. MYSM1 stabilizes the ERα protein through ERα deubiquitination.
(A, B) Western blot (A) and qPCR (B) analysis in T47D/MCF-7 cells infected with shCtrl or shMYSM1 lentivirus to evaluate the impact of MYSM1 depletion on ERα protein and mRNA levels. (C) MCF-7 cells carrying control shRNA or shMYSM1 were complemented with CHX (20 μM) for particular time periods, and cell lysates were assessed by western blot. Relative ERα level was quantified by densitometry and presented in the right plot. (D) Western blot analysis of ERα and exogenous MYSM1 expression in MCF-7/HEK293 cells transfected with wild-type MYSM1 plasmid or MPN-deletion mutant. (E) Cell lysates from MYSM1-FL/MYSM1-ΔMPN overexpressing MCF-7 cells stimulated with CHX (20 μM) at specified time points were denatured and quantitated by western blot. Relative ERα level was quantified by densitometry and presented in the right plot. (F, G) Extracts obtained from MCF-7 cells harboring shMYSM1 or FLAG-MYSM1 upon MG132 (10 μM) treatment for 4 h were analyzed by western blot. (H) MCF-7 cells from the control or the MYSM1-knockdown group were harvested after MG132 (10 μM) addition for 6 h, followed by immunoprecipitation with anti-ERα and subsequently probed with anti-His. (I) Immunoblot analysis of the polyubiquitination of ERα proteins in MCF-7 cells transiently co-transfected with plasmids encoding ERα and FLAG-MYSM1 or MYSM1-ΔMPN. MG132 (10 μM) was added to cell culture 6 h before cell collection. Cell lysates were subjected to immunoprecipitation with anti-ERα and immunoblot with anti-His. (J, K) HEK293 cells transfected with the indicated plasmids were extracted and immunoprecipitated with anti-ERα, followed by immunoblot with anti-HA. Data information: (A–K): n = 3 independent experiments performed in duplicate. (A, B, D): mean ± SD; Student’s t test; n = 3, NS: not significant. Source data are available online for this figure.