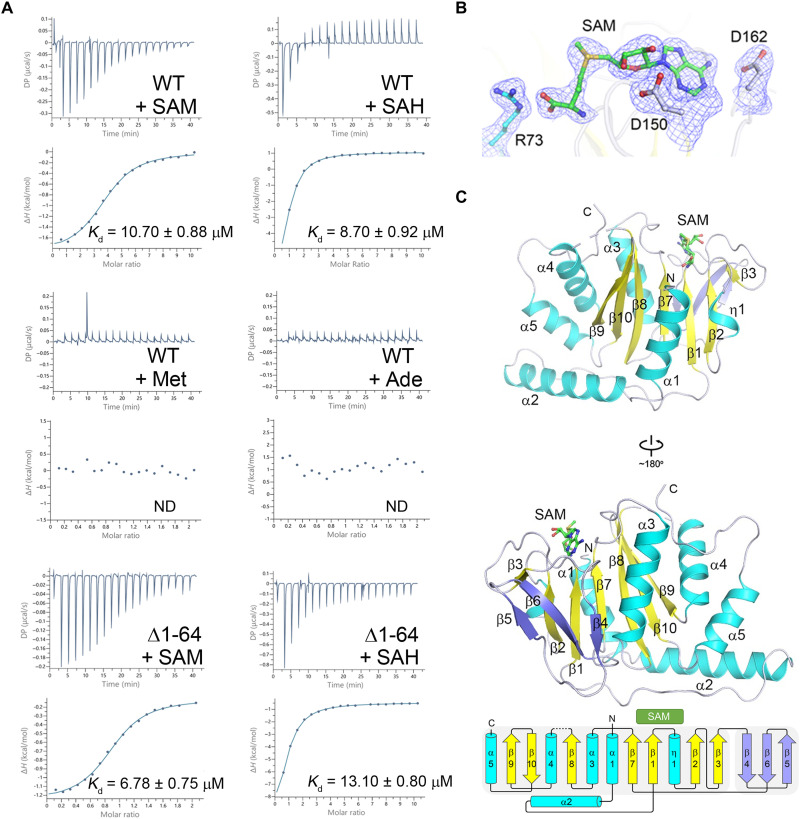
Fig. 1. SAM binds exclusively to the MTase domain of dSAMTOR.
(A) ITC measurements for the ligand-binding affinity of the WT and the Δ1-64 mutant dSAMTOR. ND, not detected. The experiments were performed three times for those with measurable binding and two times for those with undetectable binding, and the repeated experiments yielded similar results; for each case, only the result of one representative experiment is shown. (B) Composite simulated annealing Fo − Fc omit map (contoured at 1.5 σ) for the bound SAM and several surrounding residues. (C) Overall structure of the SAM-bound MTase domain of dSAMTOR in two different views. The α helices, major β sheet, and minor β sheet are colored in cyan, yellow, and blue, respectively. The bound SAM is shown with a stick model in green. The topology of the secondary structure elements of the MTase domain is shown below. The linker between β8 and α4 (residues 232 to 238) is disordered and thus is shown with a dashed line.